Why Ammonia?
The most energy dense fuel on gravimetric basis is pure hydrogen with around 120 MJ/kg. It is also the cleanest fuel rejecting only water in a combustion reaction with oxygen. However, on volumetric basis one litre of liquid hydrogen only contains around 8 MJ which is about a quarter of petrol. It is also very expensive and energy intensive to store and transport pure hydrogen in liquid state. A more affordable approach storing hydrogen in gaseous state at 350bar has an even lower volumetric energy density of lousy 3MJ/litre. [1]
At this point ammonia comes into consideration because it is the best hydrogen carrier beside hydrogen itself with a volumetric energy density of 11.5 MJ/litre in anhydrous liquid state. On its weight basis Ammonia consists of 18% hydrogen with an energy density of 22.5 MJ/kg. The biggest advantage though is that ammonia can be stored in liquid state at 25°C and moderate 10bar in standard steel tanks which are already used for liquified petroleum gas. [2]
Furthermore, ammonia has been already used for a century as refrigerant or in the fertilizer industry, being one of the most produced chemicals in the world. In other words, the industry knows very well how to deal with ammonia as a toxic substance for human and experience has shown that there is no great disadvantage compared to likewise toxic fossil fuels. Even if there occur leakages, due to its lower density than air, it dissipates quickly and people are only affected in the immediate surrounding.
Due to its fairly good energy density, ammonia can be either combusted in an engine or in a fuel cell. Particularly since ammonia does not contain any carbon atoms, there are no harmful carbon emissions at all. Potential NOx emissions in combustion engines can be avoided by lower combustion temperatures. All this promising facts show that our entire energy system could rely on ammonia without great environmental impacts or technical obstacles.
At this point ammonia comes into consideration because it is the best hydrogen carrier beside hydrogen itself with a volumetric energy density of 11.5 MJ/litre in anhydrous liquid state. On its weight basis Ammonia consists of 18% hydrogen with an energy density of 22.5 MJ/kg. The biggest advantage though is that ammonia can be stored in liquid state at 25°C and moderate 10bar in standard steel tanks which are already used for liquified petroleum gas. [2]
Furthermore, ammonia has been already used for a century as refrigerant or in the fertilizer industry, being one of the most produced chemicals in the world. In other words, the industry knows very well how to deal with ammonia as a toxic substance for human and experience has shown that there is no great disadvantage compared to likewise toxic fossil fuels. Even if there occur leakages, due to its lower density than air, it dissipates quickly and people are only affected in the immediate surrounding.
Due to its fairly good energy density, ammonia can be either combusted in an engine or in a fuel cell. Particularly since ammonia does not contain any carbon atoms, there are no harmful carbon emissions at all. Potential NOx emissions in combustion engines can be avoided by lower combustion temperatures. All this promising facts show that our entire energy system could rely on ammonia without great environmental impacts or technical obstacles.
How to produce Ammonia?
The ammonia synthesis or Haber-Bosch process was invented by the German chemists Fritz Haber and Carl Bosch in 1909. Nowadays the fertilizer industry still uses this process primarily based on natural gas to produce large quantities at affordable costs. However, the production of ammonia is also possible by using hydrogen from the water electrolysis. To make the process sustainable the electricity demand for the electrolysis must be entirely from renewable energy resources such as wind or solar energy.
 [4]
[4]
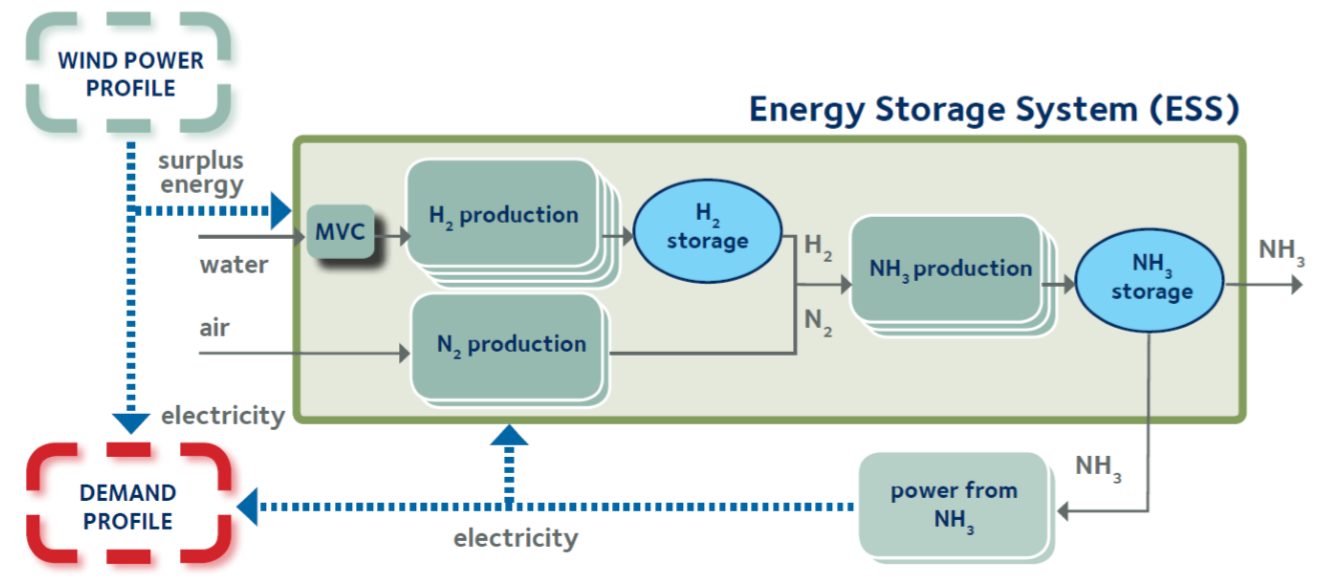
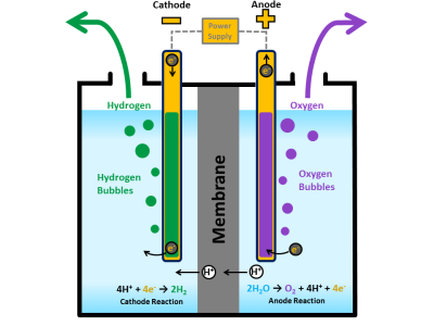
The overall process of producing ammonia and utilizing it again is shown in the schematic above. Prior to the electrolyser unit, water needs to be purified in a mechanical vapour compression unit (MVC). Thereafter, the electrolysis splits water to produce hydrogen and oxygen. The positive charged anode and the negative charged cathode are separated by a solid electrolyte material i.e. the most common used is a polymer electrolyte membrane (PEM) made of a special plastic. At the anode water reacts to oxygen and hydrogen ions. The positive charged hydrogen ions move through the electrolyte to the cathode, where they react with electrons to hydrogen gas. [4]
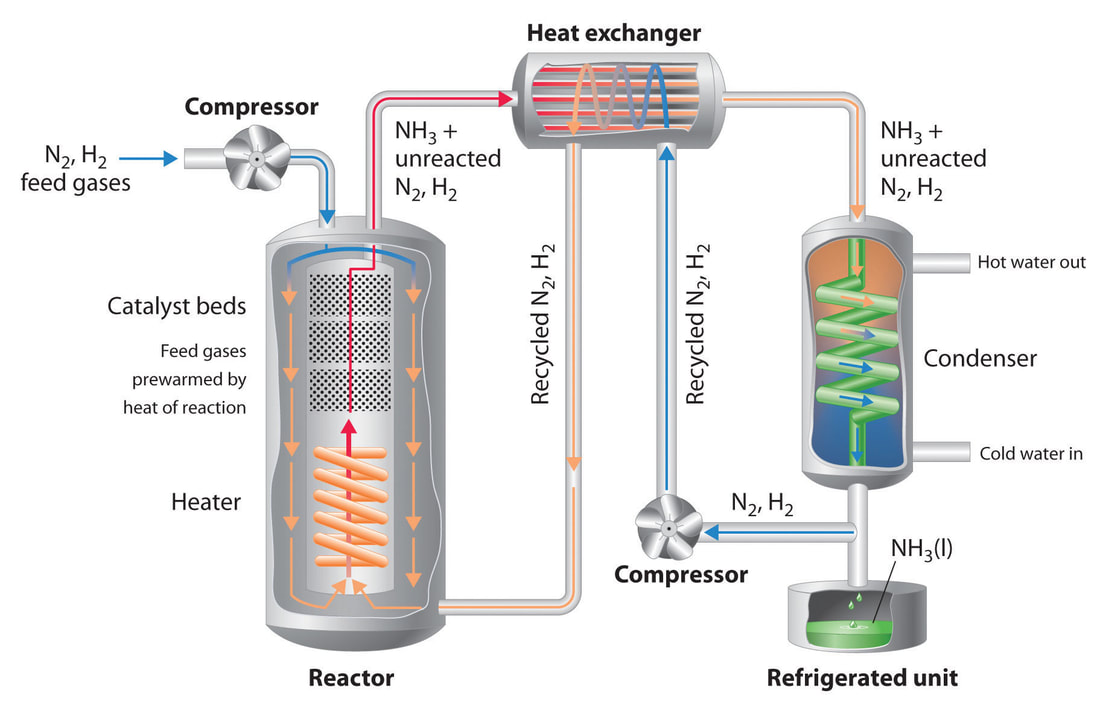
The hydrogen gas from the electrolysis eventually passes into the ammonia synthesis reactor where it reacts under high pressure and high temperature with nitrogen. Nitrogen is abundant in the air and can be extracted by distillation or pressure swing adsorption. The ammonia synthesis is an exothermic reaction using an iron based catalyst to keep the reaction efficient. Standard reaction conditions are pressures between 200bar to 400bar and temperatures between 400°C to 650°C [5]. Eventually, ammonia is compressed to liquid state and can be stored.
The hydrogen gas from the electrolysis eventually passes into the ammonia synthesis reactor where it reacts under high pressure and high temperature with nitrogen. Nitrogen is abundant in the air and can be extracted by distillation or pressure swing adsorption. The ammonia synthesis is an exothermic reaction using an iron based catalyst to keep the reaction efficient. Standard reaction conditions are pressures between 200bar to 400bar and temperatures between 400°C to 650°C [5]. Eventually, ammonia is compressed to liquid state and can be stored.
How to store Ammonia?
|
Assuming an energy density of 11.5 MJ/litre, a single storage tank of 40,000m³ (as shown in the picture) can store enough energy to meet the annual electricity demand of 30,000 households. Although the storage tanks do not require any special materials ammonia has a high coefficient of thermal expansion. The volumetric density at saturation pressure and 0°C is 636 kg/m³ whereas at 20°C it reduces to 609 kg/m³ [7]. Therefore, tank walls need to be insulated to avoid material stress and potential bursting.
|
Ammonia to Power
 [9]
[9]
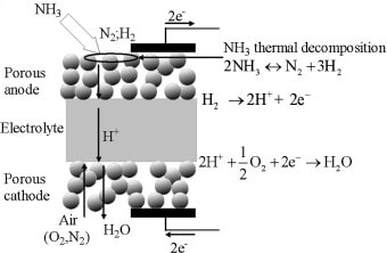
Once ammonia is stored it can be used as a product for agricultural fertilizers, cooling liquids or as a fuel for power generation. The most promising technology of using ammonia directly as a fuel is the solid oxide fuel cell (SOFC). Same as the electrolysis the fuel cell consists of the three sections cathode, electrolyte and anode. Nitrogen itself can not be used in the cell, that is why ammonia needs to be thermally decomposed to pure hydrogen and nitrogen. Hydrogen releases electrons and protons at the anode-electrolyte interface. Protons pass through the electrolyte and combine at the cathode-electrolyte interface with oxygen and electrons to water. At the same time electrons are transported through an external circuit to yield electricity [9].
[1] https://research-repository.griffith.edu.au/bitstream/handle/10072/17814/44366_1.pdf?sequence=2
[2] http://www.elgas.com.au/blog/1969-how-much-pressure-is-in-lpg-propane-cylinders-in-what-state
[3] http://www.eng.ox.ac.uk/systemseng/publications/Ammonia-based_ESS.pdf
[4] https://www.energy.gov/eere/fuelcells/hydrogen-production-electrolysis
[5] https://www.britannica.com/technology/Haber-Bosch-process
[6] http://haber-boschprocess.blogspot.co.uk/
[7] https://www.engineeringtoolbox.com/ammonia-liquid-thermal-properties-d_1765.html
[8] http://www.safichemgroup.com/en/segments/manufacturing-industry-3/areas/storage-tank-facilities-10/
[9] https://www.sciencedirect.com/science/article/pii/S0360319908008768
Resource picture: https://www.basf.com/images/corp/news-media/science-around-us/how-rotor-blades-defy-the-forces-of-nature/P-10-224_Wind_turbine_Antwerpen.jpg
[2] http://www.elgas.com.au/blog/1969-how-much-pressure-is-in-lpg-propane-cylinders-in-what-state
[3] http://www.eng.ox.ac.uk/systemseng/publications/Ammonia-based_ESS.pdf
[4] https://www.energy.gov/eere/fuelcells/hydrogen-production-electrolysis
[5] https://www.britannica.com/technology/Haber-Bosch-process
[6] http://haber-boschprocess.blogspot.co.uk/
[7] https://www.engineeringtoolbox.com/ammonia-liquid-thermal-properties-d_1765.html
[8] http://www.safichemgroup.com/en/segments/manufacturing-industry-3/areas/storage-tank-facilities-10/
[9] https://www.sciencedirect.com/science/article/pii/S0360319908008768
Resource picture: https://www.basf.com/images/corp/news-media/science-around-us/how-rotor-blades-defy-the-forces-of-nature/P-10-224_Wind_turbine_Antwerpen.jpg