Assumptions
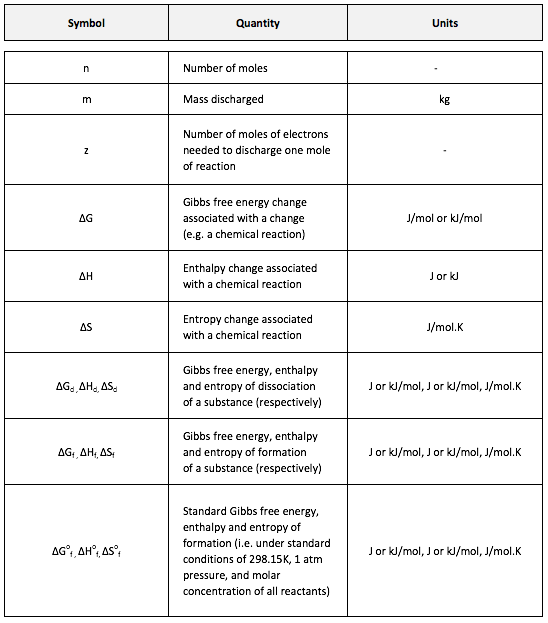
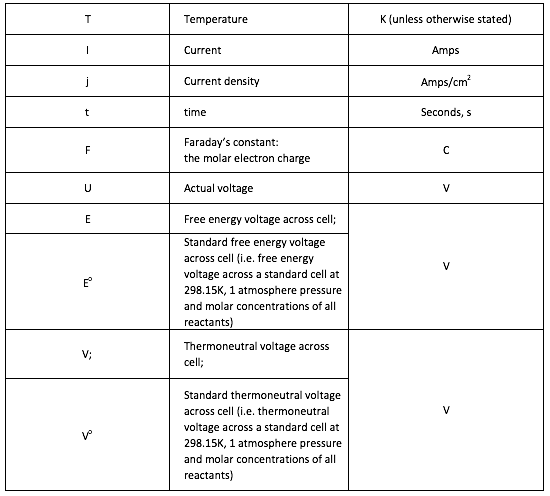
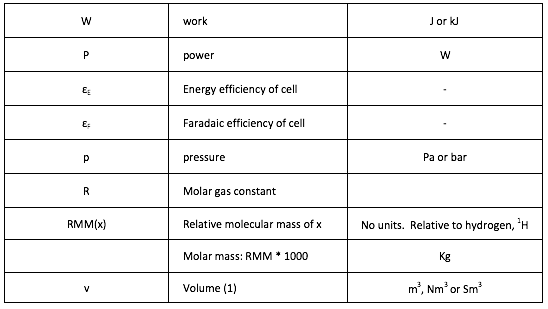
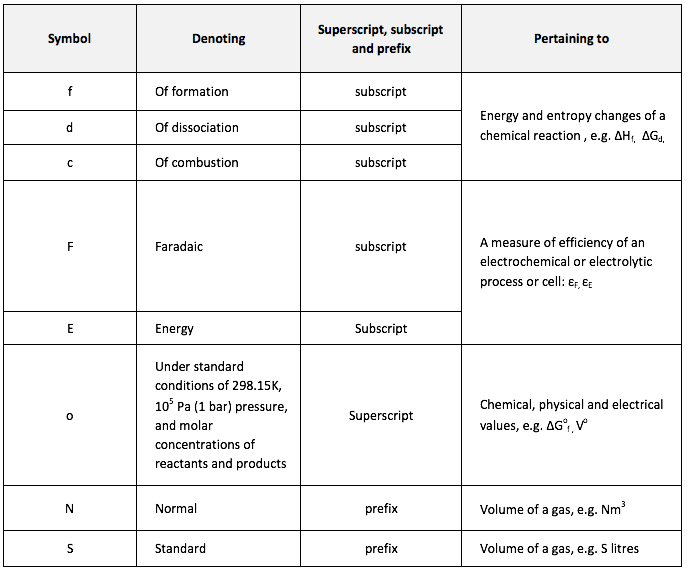
Symbols
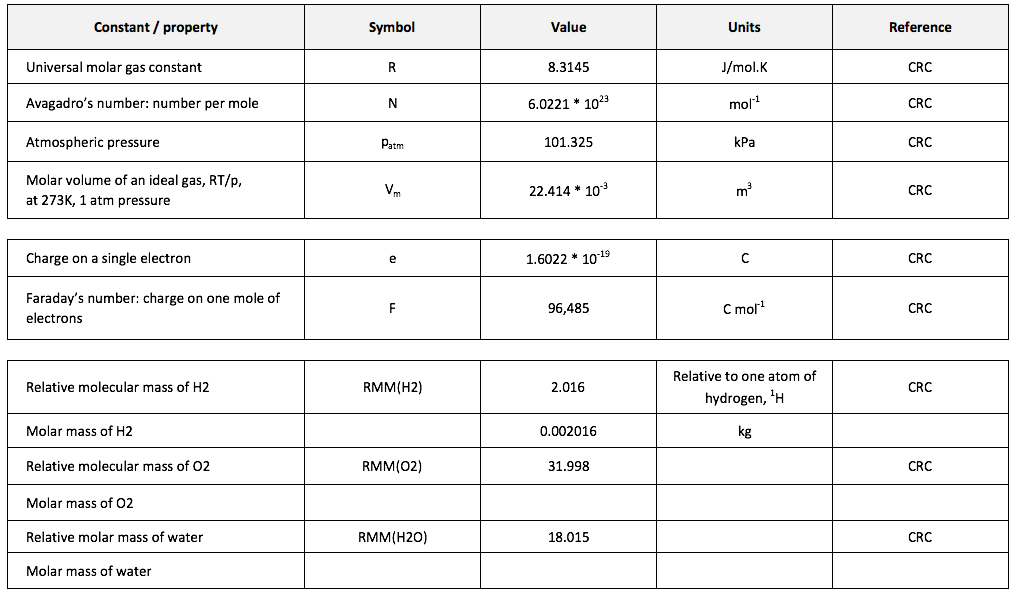
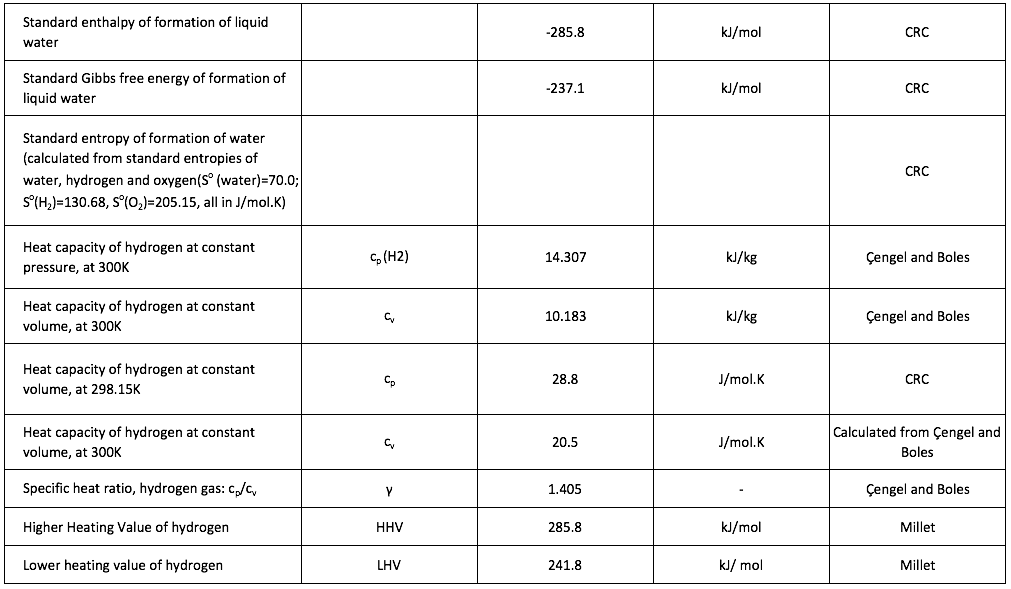
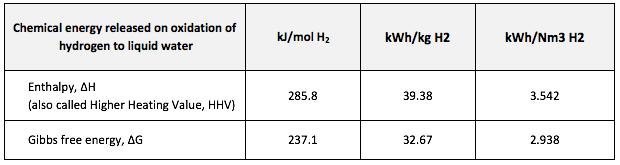
[1] The volume of a gas is often stated in “Normal metres cubed, Nm3”, which is the volume the gas would occupy at 273.15K and 1 atmosphere pressure, or “Standard metres cubed, Sm3” which is the volume the gas would occupy at 288.15K and 1 atmosphere pressure.
|
H3P PROJECT - Modular Peak Power Plant