|
|
A
very important matter for all the fuel cell types is the concentration of the
electrolyte. The higher the concentration the higher the efficiency of the fuel
cell. Therefore, different concentrations of Potassium Hydroxide, which was the
electrolyte, were used.
The
first experiment involved the use of four-mole concentration of potassium
hydroxide while the following experiments carried out with two-mole
concentration, one-mole concentration and finally half-a-mole concentration.
The
required equipment for this kind of experiment is shown on the photo below.

One
100mohm variable resistor, a thermometer with a thermocouple, a multimeter, and
wires with connectors used.
Once
the kit was set up, by altering the variable resistor different values of the
voltage could be reached. For every different value of the voltage the power
output was recorded. Therefore the peak power could exactly be obtained and
examined.
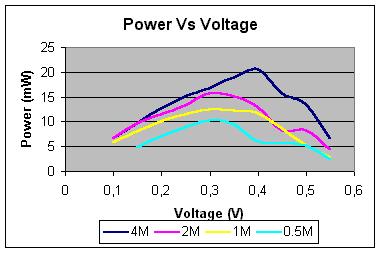
The values retrieved from that
experiments are all summarised on the following tables. These tables demonstrate
the power output behaviour for different values of the voltage.
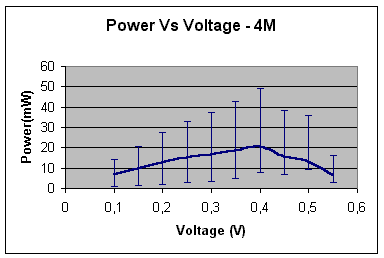
Hence, in the case of 4 –
mole concentration solution of potassium hydroxide:
2 – mole concentration:
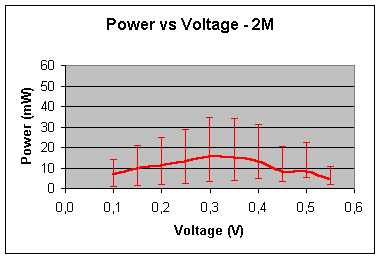
Maximum
power (mW) »
18 At 0.3V
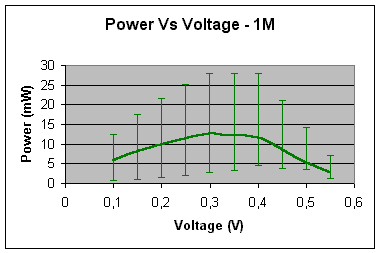
1 – mole concentration
0.5 – mole
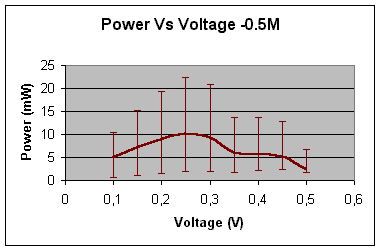
Maximum power
(mW) »
10 At 0.25V
Comparing all the results:
Maximum power
(mW) »
14 At 0.3V