It
is widely known that energy in the form of heat can easily speed – up chemical
reactions. Therefore, this part of the
web page refers to the effect of temperature under constant electrical load to
the fuel cell operation.
Once
more the equipment used was the same. A methanol fuel cell kit was operated.

Hence, by setting the voltage
constant to a certain value, by the help of the variable resistor and by taking
measurements of the power at different temperatures the following table
constructed:
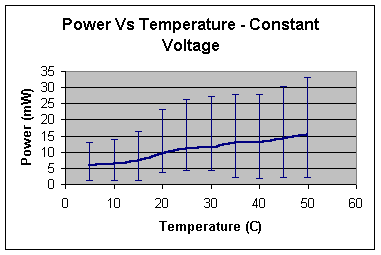
As
someone may notice from the table, the higher temperature during the experiment
was 50 °C
while the lower was only 5-6 °C.
The
power output turned out to reach higher values as temperature rose.
From
the almost linear graph above someone may assume that the fuel cell reaction
would continue to rise with further increases in temperature.