Environmental Considerations of Biomass Combustion
Introduction
This section will cover the environmental impacts which can arise from the utilisation of solid ‘woody’ biomass as a fuel source. Impacts associated with the combustion of liquid biomass will be covered within the anaerobic digestion analysis for Barony College while impacts from energy crops are not covered since they are not yet widespread in Scotland.
If biomass is to be seen as a credible ‘green’ alternative to the utilisation of fossil fuels the potential environmental and health impacts which arise from its combustion must be managed. Legal compliance must also be taken into account. It should be noted there are wider environmental considerations which need to be understood if large scale utilisation of biomass is to occur apart from those solely associated with combustion. These will be covered in brief.
Biomass Fuel Analysis
To gain a greater understanding of the combustion products of solid biomass we must first consider its composition. Under analysis it is found that the basic constituents of ‘woody’1 plant species are as follows: -
• Cellulose.
• Hemi-cellulose.
• Lignin
• Water (circa 60% weight).
• Extraneous Compounds.
Lignin is a highly cross linked insoluble phenolic polymer, the structure of which is species dependant. Water is stored in the tree either ‘free’ inside the hollow centre of cells or ‘bound’ inside the cell wall bound to cellulose or hemi-cellulose. When the wood fuel is dried the moisture content (MC) equilibrates with the ambient relative humidity. The percentage composition of the above five components differs between softwood and hardwood species; the former being predominantly used in the wood processing industry.
As regards environmental considerations the extraneous compounds are of particular interest as these include inorganic2 compounds, including toxic heavy metals such as cadmium, lead and mercury. These extraneous compounds account for 2-5% of wood composition and levels are dependant on the species of plant, its age and proximity of pollution sources.
All biomass sources contain sulphur (.01-.03%), nitrogen and chlorine. Levels of the first two however are far lower than is found within coal. The concentrations of these elements are lower in wood than energy crops or straw. The presence of chlorine serves to mobilise inorganic compounds, especially potassium, and is therefore a major factor in ash and dioxin formation. The latter will occur especially if the wood has been treated with organo-chlorine wood preservative or PVC. Potassium and Sodium are present in higher concentrations within biomass than coal however.
Basic Combustion Outline
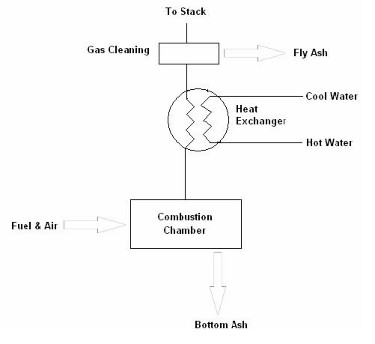
The following diagram is a very basic overview of where combustion by-products arise in a heat only biomass system: -
Fig 1. Wastes from Combustion Process (reproduced from ‘Royal Commission on Environmental Pollution’, 2004).
Ash Production
Ash is the inorganic mineral residue remaining after wood has been pyrolised in an oxygen atmosphere. The volume of ash produced is primarily dependant on the species of biomass; softwoods are typically 1% dry weight while plant material ranges from 6-16%. Other factors which influence its production are thermal conductivity, MC and particle size of the fuel. A generic particle size specification for biomass is .6cm but this will vary with system utilised3.
The bottom ash and fly ash produced are of different compositions and therefore disposed of differently. Most non-combustible minerals leave as bottom ash. The PH of bottom ash varies from neutral and alkaline. Since its main components are potassium and phosphorous in most cases it is suitable to be used as fertiliser or as an aggregate in construction. Analysis should be conducted prior to utilising in this manner however.
The fly ash is of a smaller volume and requires specialist disposal. This is due to the presence of toxic heavy metals, dioxins and furans. The combustion process effectively concentrates these undesirable elements so they can be disposed of. The concentrations of heavy metals present in fly ash increase as the precipitation temperature and particle size are reduced. These pollutants and detrimental substances are mainly dust bound. Therefore in order to ensure conformance with environmental legislation and avoid health risks gas cleaning technology is required. Toxic elements and particulates can be removed by various methods such as adsorption4, post precipitation (to remove dust) and ion exchange. Generally the higher the ash content the less desirable the fuel source.
From an environmental perspective there may be unburned hydrocarbons and soot present in the combustion chamber if the process is not performed efficiently; however this is mainly associated with lower temperature small biomass combustion systems.
Gas Emissions
The following two equations, one for photosynthesis5 and one for combustion, show the rationale behind the classification of biomass as a ‘carbon neutral’ fuel source i.e. the carbon dioxide released is equal to that sequestered during growth.
Photosynthesis: CO2 + 2H2O
—>CH2O + O2 + H2O
Combustion: Cx Hy Oz + (x + y / 4 –
z / 2) O2—> xCO2 + (y / 2) H2O
The main combustion products therefore, as shown by the combustion reaction, are the greenhouse gas carbon dioxide and water. It should be noted though that additional carbon dioxide will be brought into the cycle from activities such as mechanised harvesting, transport (estimated at .18 -.27 kg CO2/odt/km6 dependant on fuel type) and processing i.e. chipping/pelletisation. For example the latter requires compression and then energy intensive heating to melt lignin and effectively ‘glue’ the pellet together.
Furthermore the utilisation cycle of biomass feedstock can also result in emissions of other greenhouse gases such as methane (CH4) and nitrous oxide (N2O) which hold more potent climate change potential than CO2. The water is usually emitted as a harmless steam plume; concerns over a ‘visual impact’ can be negated through utilising a condenser or by re-heating.
There are other gaseous emissions to consider however. Oxides of Nitrogen and Sulphur (NOx & SOx) will also be produced . The concentration of these gases produced largely depends on the chemical composition of the fuel; however the lower combustion temperatures typically associated with biomass decreases NOx production further. The latter is mainly sourced from the nitrogen present in the air rather than the fuel itself. Acidic gases such as Hydrogen Chloride (HCl), a contributor to acid rain, can also be emitted. The injection of lime powder can be utilised to counter this.
The fossil fuel which is replaced by biomass will determine if the extent to which emissions are increased or decreased. For example: -
• Coal replaced with Biomass: SO2, CO, Particulate Matter (PM), Non-Methane Volatile Organic Carbon (NMVOC’s) are all decreased.
• Oil replaced with Biomass: SO2 reduced but PM, NOx increased.
• Gas replaced with Biomass: An increase in all airborne emissions is observed.
Table 1 (below) highlights the main emissions differences associated with biomass combustion when compared with fossil fuels.
Pollutant |
Advantage
'+' or Disadvantage '-' from change to modern biomass technology
(wood) from fossil fuel |
||
Gas |
Oil |
Coal |
|
SO2 |
-- |
++ |
+++ |
NOx |
- |
- |
+ |
PM/PM10/PM2.5 |
--- |
-- |
+ |
CO |
- |
- |
+ |
nmVOC |
- |
- |
+ |
Trace
Elements |
-- |
+ |
+ |
PAH |
-- |
- |
+ |
PCDD/F |
-- |
- |
+ |
Table 1: Emissions Comparison. (Scottish Executive, 2006.)
It also needs to be considered that if combustion is inefficient carbon monoxide (CO) will be emitted, the completeness of combustion being determined by factors such as the chamber temperature, turbulence gases and oxygen excess. The efficiency of biomass combustion has increased dramatically however with modern plants releasing less than 100mg CO/M³8. The permitted average limit is 150ppm. Hydrocarbons and Volatile Organic Carbons will also be produced from incomplete combustion.
The total organic carbon (Corg) content
of the flue gas can also be utilised as a proxy for combustion quality
and overall emissions of harmful/carcinogenic substances such as formaldehyde
and phenols. Flue gases high in Corg will also cause nuisance smells.
As mentioned when referring to fly ash, flue gases will hold particulate
matter, the volume dependant on the completeness of combustion. These
particulates serve as condensation points for organic and trace inorganic
elements; which are carcinogenic and should be removed as far as possible.
Wider Environmental Considerations
Carbon Dioxide is stored in soil and vegetation. “Carbon Sequestration can be defined as the accumulation of carbon in a particular ecosystem or land use system” (Scottish Executive, 2006). If carbon is stored in these environments they are termed ‘sinks’ while if it is released they are designated as ‘sources’. It should be noted that a large scale switch to biomass could result in land use changes which act as sources or sinks, depending on what ecosystem is replaced.
Pollutant emissions such as SOx, NOx and HCl can contribute to acid rain which in turn will acidify soils and water where it falls and therefore damage plant, animal and aquatic ecosystems. Trees will also be damaged through affects to their leaves and certain types of building stones partially dissolved.
It has been hypothesised (Scottish Executive, 2006) that the removal of agricultural and forestry residues from existing land use may deplete the carbon content of soil, through a reduction in the organic matter added, the result of this can be detrimental through increasing the prospect of erosion and reducing water quality by increased sedimentation of water courses.
According to Brierley et al (2004, cited Scottish Executive 2006) the extent of the impact caused as a result of removing forestry residues will be dependant on the mode of removal, intensity of the operation and soil type. The use of brash mats can increase erosion and promote excessive nutrient depletion while heavy harvesting machinery can result in soil compaction.
The effects of biomass extraction for energy on in-situ biodiversity depend on land use transition which has taken place coupled with the intensity of cultivation. Bengsson et al (1998, cited Scottish Executive, 2006) studied two sites where one was left undisturbed and in the other residues were removed. A drop in invertebrate fauna in the region of 30-60% was observed in the latter. It is further predicted that extensive brash removal will deprive many species of fauna and fungi of habitat and food sources. This should be balanced however with the positive impacts arising from forest thinning.
The prospect of increased congestion due to regular fuel deliveries should also be considered.
Footnotes
- This does not just denote trees, many shrubs and fast growing plants utilised in short rotation coppicing (SRC) i.e. with high wood to foliage ratio, are covered by this description.
- Does not contain a Carbon chain
- Demirbas, A. (2005).
- Using activated Carbon
- The organic synthesis from atmospheric carbon dioxide by means of photo energy
- Forum for Renewable Energy Development in Scotland, 2005
- Principally NO and SO
- Huber, S. & Friels, H., 2006
References
Demirbas, A., 2005. Potential Applications of renewable Energy Sources, Biomass Combustion Problems in Boiler Power Systems and Combustion Related Environmental Issues. Progress in Energy and Combustion Science, 31, 171-192.
The Royal Commission on Environmental Pollution, 2004. ‘Biomass as a Renewable Energy Source.’ London: The Royal Commission on Environmental Pollution.
AEA Technology, 2005. ‘Renewable Heat and Heat from Combined Heat and Power Plants – Study & Analysis.’ London: Department of Trade & Industry.
Forum for Renewable Energy Development in Scotland, 2005. ‘Promoting and Accelerating the Market Penetration of Biomass Technology in Scotland,’ Edinburgh: Scottish Executive.
Scottish Executive Environmental & Rural Affairs Committee, 2006. ‘Review of Greenhouse Gas life Cycle Emissions, Air Pollution Impacts and Economics of Biomass Production and Consumption in Scotland.’ Edinburgh: Scottish Executive.
Kitani, O.S. & Hall, C.W. eds., 1989. ‘Biomass Handbook.’ New York: Gordon & Branch Publishers.
Huber, S. & Friels, H., 2006. ‘Emissions of Biomass Combustion Plants’ [online]. Ausberg, Bavarian State Office for Environmental Protection.