
 |
| Rapeseed fields produce vivid colours |
Biodiesel is an alternative fuel similar to conventional or ‘fossil’ diesel. Biodiesel can be produced from straight vegetable oil, animal oil/fats, tallow and waste cooking oil. The process used to convert these oils to Biodiesel is called transesterification. This process is described in more detail below. The largest possible source of suitable oil comes from oil crops such as rapeseed, palm or soybean. In the UK rapeseed represents the greatest potential for biodiesel production. Most biodiesel produced at present is produced from waste vegetable oil sourced from restaurants, chip shops, industrial food producers such as Birdseye etc. Though oil straight from the agricultural industry represents the greatest potential source it is not being produced commercially simply because the raw oil is too expensive. After the cost of converting it to biodiesel has been added on it is simply too expensive to compete with fossil diesel. Waste vegetable oil can often be sourced for free or sourced already treated for a small price. (The waste oil must be treated before conversion to biodiesel to remove impurities). The result is Biodiesel produced from waste vegetable oil can compete with fossil diesel. More about the cost of biodiesel and how factors such as duty play an important role can be found here.
What are the benefits of Biodiesel?Biodiesel has many environmentally beneficial properties. The main benefit of biodiesel is that it can be described as ‘carbon neutral’. This means that the fuel produces no net output of carbon in the form of carbon dioxide (CO2). This effect occurs because when the oil crop grows it absorbs the same amount of CO2 as is released when the fuel is combusted. In fact this is not completely accurate as CO2 is released during the production of the fertilizer required to fertilize the fields in which the oil crops are grown. Fertilizer production is not the only source of pollution associated with the production of biodiesel, other sources include the esterification process, the solvent extraction of the oil, refining, drying and transporting. All these processes require an energy input either in the form of electricity or from a fuel, both of which will generally result in the release of green house gases. To properly assess the impact of all these sources requires use of a technique called life cycle analysis. Our section on LCA looks closer at this analysis. Biodiesel is rapidly biodegradable and completely non-toxic, meaning spillages represent far less of a risk than fossil diesel spillages. Biodiesel has a higher flash point than fossil diesel and so is safer in the event of a crash.
Biodiesel ProductionAs mentioned above biodiesel can be produced from straight vegetable oil, animal oil/fats, tallow and waste oils. There are three basic routes to biodiesel production from oils and fats:
Almost all biodiesel is produced using base catalyzed transesterification as it is the most economical process requiring only low temperatures and pressures and producing a 98% conversion yield. For this reason only this process will be described in this report.
The Transesterification process is the reaction of a triglyceride (fat/oil) with an alcohol to form esters and glycerol. A triglyceride has a glycerine molecule as its base with three long chain fatty acids attached. The characteristics of the fat are determined by the nature of the fatty acids attached to the glycerine. The nature of the fatty acids can in turn affect the characteristics of the biodiesel. During the esterification process, the triglyceride is reacted with alcohol in the presence of a catalyst, usually a strong alkaline like sodium hydroxide. The alcohol reacts with the fatty acids to form the mono-alkyl ester, or biodiesel and crude glycerol. In most production methanol or ethanol is the alcohol used (methanol produces methyl esters, ethanol produces ethyl esters) and is base catalysed by either potassium or sodium hydroxide. Potassium hydroxide has been found to be more suitable for the ethyl ester biodiesel production, either base can be used for the methyl ester. A common product of the transesterification process is Rape Methyl Ester (RME) produced from raw rapeseed oil reacted with methanol.
The figure below shows the chemical process for methyl ester biodiesel. The reaction between the fat or oil and the alcohol is a reversible reaction and so the alcohol must be added in excess to drive the reaction towards the right and ensure complete conversion.
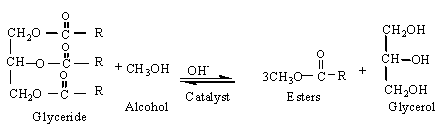
A successful transesterification reaction is signified by the separation of the ester and glycerol layers after the reaction time. The heavier, co-product, glycerol settles out and may be sold as it is or it may be purified for use in other industries, e.g. the pharmaceutical, cosmetics etc.
Straight vegetable oil (SVO) can be used directly as a fossil diesel substitute however using this fuel can lead to some fairly serious engine problems. Due to its relatively high viscosity SVO leads to poor atomisation of the fuel, incomplete combustion, coking of the fuel injectors, ring carbonisation, and accumulation of fuel in the lubricating oil. The best method for solving these problems is the transesterification of the oil.
The engine combustion benefits of the transesterification of the oil are:
An example of a simple production flow chart is proved below with a brief explanation of each step.
(ref 1)
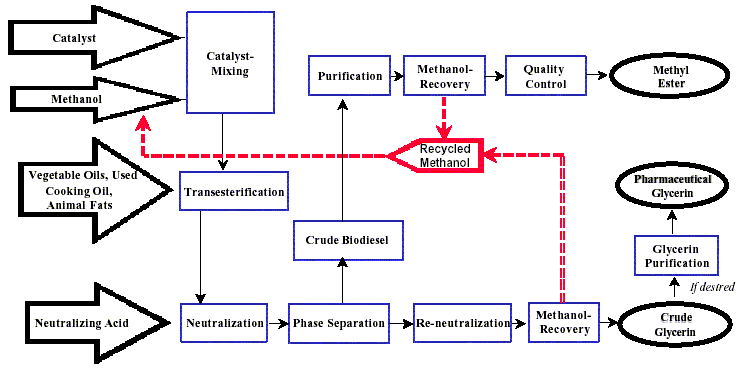
Mixing of alcohol and catalyst
The catalyst is typically sodium hydroxide (caustic soda) or potassium hydroxide (potash).
It is dissolved in the alcohol using a standard agitator or mixer. Reaction. The alcohol/catalyst mix is then charged into
a closed reaction vessel and the oil or fat is added. The system from here on is totally closed to the atmosphere to
prevent the loss of alcohol. The reaction mix is kept just above the boiling point of the alcohol (around 160 °F) to speed
up the reaction and the reaction takes place. Recommended reaction time varies from 1 to 8 hours, and some systems
recommend the reaction take place at room temperature. Excess alcohol is normally used to ensure total conversion of the
fat or oil to its esters. Care must be taken to monitor the amount of water and free fatty acids in the incoming oil or fat.
If the free fatty acid level or water level is too high it may cause problems with soap formation and the separation of
the glycerin by-product downstream.
Separation
Once the reaction is complete, two major products exist: glycerin and biodiesel. Each has a substantial amount of
the excess methanol that was used in the reaction. The reacted mixture is sometimes neutralized at this step if needed.
The glycerin phase is much more dense than biodiesel phase and the two can be gravity separated with glycerin simply
drawn off the bottom of the settling vessel. In some cases, a centrifuge is used to separate the two materials faster.
Alcohol Removal
Once the glycerin and biodiesel phases have been separated, the excess alcohol in each phase is removed with a
flash evaporation process or by distillation. In others systems, the alcohol is removed and the mixture neutralized before
the glycerin and esters have been separated. In either case, the alcohol is recovered using distillation equipment and
is re-used. Care must be taken to ensure no water accumulates in the recovered alcohol stream.
Glycerin Neutralization
The glycerin by-product contains unused catalyst and soaps that are neutralized with an acid and sent to storage
as crude glycerin. In some cases the salt formed during this phase is recovered for use as fertilizer. In most cases
the salt is left in the glycerin. Water and alcohol are removed to produce 80-88% pure glycerin that is ready to be sold
as crude glycerin. In more sophisticated operations, the glycerin is distilled to 99% or higher purity and sold into the
cosmetic and pharmaceutical markets.
Methyl Ester Wash
Once separated from the glycerin, the biodiesel is sometimes purified by washing gently with warm water to remove
residual catalyst or soaps, dried, and sent to storage. In some processes this step is unnecessary. This is normally
the end of the production process resulting in a clear amber-yellow liquid with a viscosity similar to petrodiesel.
In some systems the biodiesel is distilled in an additional step to remove small amounts of color bodies to produce a
colorless biodiesel.
Product Quality
Prior to use as a commercial fuel, the finished biodiesel must be analyzed using sophisticated analytical equipment
to ensure it meets any required specifications. The most important aspects of biodiesel production to ensure trouble
free operation in diesel engines are: