|
Radon
gas occurs naturaly by the radioactive dacay of Uranuim, which is present
in small quantities underneath houses and in some building materials.
The gas, like Carbon Monoxide, is colourless, odourless and tasteless
but has been identified as the second largest cause of lung cancer after
smoking. Radon levels vary widely in the UK, but the gas is particularly
prevalent in areas of granite or limestone. Epecially where these rocks
make up the building materials, for example, in “the Granite City” of
Aberdeen.
Concentrations in the open air are very low. Radon in
soil and rocks mixes with air and rises to the surface where it is quickly
diluted in the atmosphere. However, Radon that enters enclosed spaces
and buildings, can reach relatively high concentrations in some circumstances,
especially in buildings with insufficient ventilation.
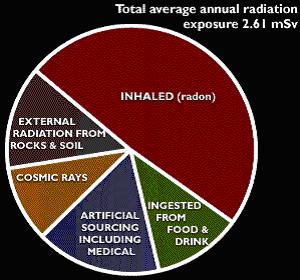
Half of the average human radiation exposure in the UK is attributable
to Radon
Health
Effects of Radon Inhalation.
Breathing high concentrations of radon can cause lung
cancer. The risk is neverless small because the gas is radiologically
not very active, with a half-life of 4 days. Unfortunately, the decay
products of radon itself are more hazardous isotopes of solid elements
with an active 30 minute half-life. These particles, such as Polonium,
irradiate the lungs more effecitvely upon inhalation and can be especially
dangerous since they can attach to other natural aerosols and subsequently
become lodged in the lining of the respiratory system. It is therefore
important to reduce indoor radon concentrations as low as reasonably
practicable.
In addition, smoking and exposure to radon are known to
work together to greatly increase the risk of developing lung cancer.
Smokers may be several times more likely to contract lung cancer from
a lifetime radon exposure at 200Bqm-3 than the general population (SOURCE:
the 6th Committee of the Biological Effects of Ionizing Radiation of
the American National Academy of Science)
It is believed that environmental radon accounts for between
2000 and 3,300 lung cancer deaths in the UK annually, which is 3 - 5%
of the total lung cancer deaths.
Radon
Detection.
Radon levels in homes vary on both a dayly and yearly
basis. This is mainly because of temperature differences between indoors
and outdoors. Concentrations of Radon are generally higher at night
and during the winter. Even in a home with good draught proofing and
double-glazing, the air changes several times a day. Increasing the
ventilation, especially on the ground floor, will in most cases cause
a moderate reduction in the radon level.
There are four main categories of detectors: -
Etched-track
detectors - The most popular and cost effective detector. Alpha particles
leave tracks in a plastic over a three-month period. The detectors are
sent to labs, processed to reveal the pitting made by alpha particles.
The pits are microscopically counted permitting a radon level to be
computed.
Electret
detectors - These can be used for measurements over periods from days
to months; operates by gauging the loss of electrostatic charge which
is neutralised by alpha particles emitted by radon and its decay products
over the period, from which radon concentration can be calculated. These
detectors are extremely delicate and must be handled carefully for accurate
results.
Charcoal
detectors - Not suitable for long-term measurement; least accurate and
used when a rapid measure is required. Activated charcoal absorbs radon,
and the laboratory can determine a rough estimate of radon concentration.
Active
monitors - The most effective and expensive Radon detector. Provides
an electronic continuous measure of radon or its decay products; permits
figures to be obtained over consecutive hours.
|