CATALYTIC
FLAMMABLE GAS SENSORS
The
concept of the pellistor is based on the fact that the most foolproof
way to determine whether a flammable gas is present in air is to test
a sample by trying to burn it. A pellistor consists of a very fine coil
of wire suspended between two posts. The coil is embedded in a pellet
of a ceramic material, and on the surface of the pellet (or 'bead')
there is a special catalyst layer.
ELECTROCHEMICAL
SENSOR TECHNOLOGY
The
carbon monoxide sensor consists of three electrodes immersed in a liquid
electrolyte (a non-metallic liquid that conducts electricity, usually
through acids or dissolved salts). The three electrodes are the working
electrode, the reference electrode, and the counter electrode. The most
important of these is the working electrode (WE). The working electrode
is made of platinum, which is a catalytic metal to CO (it catalyzes
the oxidation of CO to CO2), backed by a gas-permeable but hydrophobic
(water-proof) membrane. The CO gas diffuses through the porous membrane
and is electrochemically oxidized (Equation 1).
CO
+ H2O à CO2 + 2H+ + 2e -------------------------------------------------------(Eq.1)
The
electrons involved in the electrochemical reaction flow from the working
electrode through the external circuit, producing the output signal
of the sensor.
SEMICONDUCTOR
SENSOR’S THEORY OF OPERATION
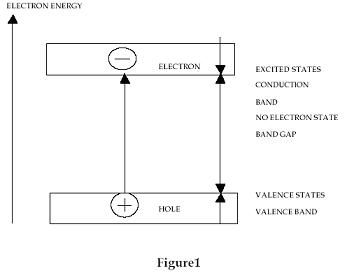
One
of the important properties of a semiconductor is the concentration
of charge carriers. As semiconductors in general contain relatively
few free charge carriers this facilitates control of their behaviour
and concentration by external means. In a clean semiconductor, negative
free electrons and positive free holes are present in equal numbers.
These electrons and holes are created by the thermal excitation of valence
electrons from the valence states, in the crystal, to the conduction
band leaving behind positive holes in the valence band. (See Fig1.)
This
is true for a so-called ‘intrinsic’ semiconductor, for a pure oxide
system, and is equally true for Silicon or Germanium. For most commercial
applications of semiconductor materials it is necessary to add a dopant,
which supplies an excess of holes or electrons that form the majority
current carrier, i.e. p or n type semiconductors. For the Chrome Titanium
oxide system, Chrome oxide is the intrinsic semiconductor, which, with
a valency state of 3+, when doped with Titanium, of valency 4+, now
becomes a p-type semiconductor. The Titanium requires an extra electron,
which it takes from the Chrome d-band leaving a hole.
The electron it takes is trapped by an oxygen atom forming
TiO2.Therefore Oxygen adsorbed at the gas–solid interface removes electrons
from the surface region of the solid to form a surface oxygen ion; the
oxygen can be thought of as a surface trap for electrons from the Chrome
oxide d-band (see Fig 2).
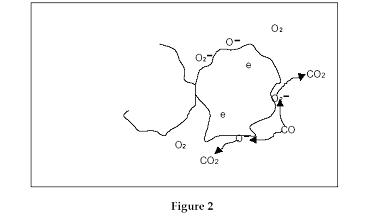
Therefore
the adsorbed oxygen gives rise to an increase in the hole concentration,
and it follows that any decrease in the surface coverage
of Oxygen ions by reacting with, for example, CO to form CO2 would release
electrons back into the lower band and decrease the hole concentration
and hence lead to an increase in the resistance of the semiconductor
material.
|