|
|
|
|
|
|
Introduction
Fuel cells are devices that can convert electrochemical energy very
efficiently and can generate electricity and produce heat, with the help of
catalysts.
Fuel cell technology is becoming increasingly popular in power generation and automotive applications. Advantages include low fuel emissions and high efficiency as it shown in the table below. The most common fuel used in a fuel cell is hydrogen
|
FC Technology |
PAFC |
PEMFC |
SOFC |
AFC |
MCFC |
|
Electrolyte |
Phosphoric acid |
Proton exchange membrane |
Solid Metal Oxide |
Potassium Hydroxide (KOH) |
Alkaline Carbonates (CO3) |
|
Operating temperature |
150-200oC |
80oC |
800-1000oC |
150-200oC |
650oC |
|
Electrical efficiency |
35-45(%) |
42-50(%) |
45-60(%) |
40(%) |
45-55(%) |
The Basics
The basic principles of a fuel cell are similar with those of the
electrochemical batteries. The big difference is that, in the case of
batteries, the chemical energy is stored in substances located inside them.
So when the energy has been converted to electrical energy, the battery must
be thrown away (primary battery) or recharged appropriately (secondary
battery).
In a fuel cell, the chemical energy is provided by a fuel and an oxidant
stored outside the cell in which the chemical reactions take place. As long
as the cell is supplied with fuel and oxidant, electrical power can be
obtained.
Operating Principle
Electrolysis is the process where by applying electric power water is decomposed into the gaseous components oxygen and hydrogen. The fuel cell reverses the aforementioned process and takes exactly these two substances and converts them to water again. In theory the same amount of energy which has been used for the electrolysis is set free by this conversion. In practice insignificant losses are caused by different physical-chemical processes.
2 H2(g) + O2(g) → 2 H2O(liq) + Energy
So to say electric power is stored in hydrogen. Therefore we have hydrogen at our disposal in which electric power can be stored. In fuel cells we get back the electric power stored in the hydrogen. Most fuel cells are operating with air, so there is no need to store oxygen[1].

Electrolysis

Energy Production from Fuel Cell
Basic Construction
Fuel cells have a very simple structure as it is shown in the
following picture. A typical fuel cell consists of three
parts: the anode side flow channel plate, the Membrane Electrode Assembly
(MEA) and the cathode flow channel plate.
Anode and cathode serve as catalyst. The mid layer consists of a
carrier structure which absorbs the electrolyte. In different types of fuel
cells different substances are used as electrolyte. Some electrolytes are
liquid and some are solid with a membrane structure.
The MEA is the key component of all fuel cells. For the Proton Exchange
Membrane (PEM) fuel cells is composed of a proton-conducting polymer
sandwiched in between two fuel cell electrodes: the anode, where hydrogen is
oxidised; and the cathode, where oxygen from air is reduced. The flow
channel plate normally has small slots or labyrinth-styled passageways to
"guide" the gas into the fuel cell and maximise gas diffusion to the
electrode for reaction to take place.
A hydrogen fuel cell only produces about 1V of electricity. To increase the
power output of the fuel cell, hundreds of identical flow channel plates and
MEAs are stacked next to each other to form a fuel cell stack. In a fuel
cell stack, both sides of each flow channel plate have slots or flow
channels to reduce the size and weight of the stack. The plate is now called
a bipolar plate. One side of the plate is next to the anode of a fuel cell,
while the other side is next to the cathode of the neighbouring fuel cell
[2].
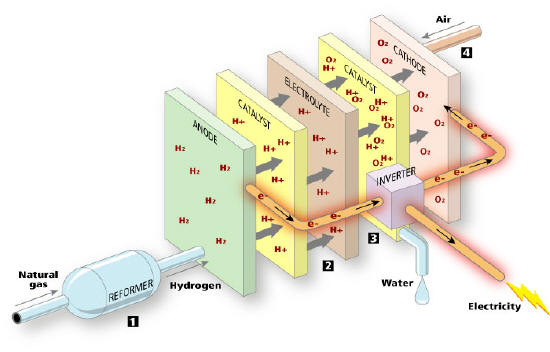
The main Parts of a Fuel Cell
References:
[1] HyNet
[2] HyNet
[3] Fuel Cell Store