|
|
|
|
|
|
Fuel cells are classified primarily by the kind of electrolyte they employ. This determines the kind of chemical reactions that take place in the cell, the kind of catalysts required, the temperature range in which the cell operates, the fuel required, and other factors. These characteristics, in turn, affect the applications for which these cells are most suitable. There are several types of fuel cells currently under development, each with its own advantages, limitations, and potential applications.
Proton Exchange Membrane Fuel Cell
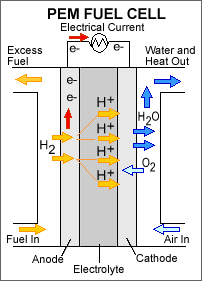 Polymer electrolyte membrane (PEM) fuel
cells—also called proton exchange membrane fuel cells—deliver high power
density and offer the advantages of low weight and volume, compared to other
fuel cells. PEM fuel cells use a solid polymer as an electrolyte and porous
carbon electrodes containing a platinum catalyst. They need only hydrogen,
oxygen from the air, and water to operate and do not require corrosive
fluids like some fuel cells. They are typically fueled with pure hydrogen
supplied from storage tanks or onboard reformers.
Polymer electrolyte membrane (PEM) fuel
cells—also called proton exchange membrane fuel cells—deliver high power
density and offer the advantages of low weight and volume, compared to other
fuel cells. PEM fuel cells use a solid polymer as an electrolyte and porous
carbon electrodes containing a platinum catalyst. They need only hydrogen,
oxygen from the air, and water to operate and do not require corrosive
fluids like some fuel cells. They are typically fueled with pure hydrogen
supplied from storage tanks or onboard reformers.
Polymer electrolyte membrane fuel cells operate at relatively low temperatures, around 80°C (176°F). Low temperature operation allows them to start quickly (less warm-up time) and results in less wear on system components, resulting in better durability. However, it requires that a noble-metal catalyst (typically platinum) be used to separate the hydrogen's electrons and protons, adding to system cost. The platinum catalyst is also extremely sensitive to CO poisoning, making it necessary to employ an additional reactor to reduce CO in the fuel gas if the hydrogen is derived from an alcohol or hydrocarbon fuel. This also adds cost. Developers are currently exploring platinum/ruthenium catalysts that are more resistant to CO.
PEM fuel cells are used primarily for transportation applications and some stationary applications. Due to their fast startup time, low sensitivity to orientation, and favorable power-to-weight ratio, PEM fuel cells are particularly suitable for use in passenger vehicles, such as cars and buses.
A significant barrier to using these fuel cells in vehicles is hydrogen storage. Most fuel cell vehicles (FCVs) powered by pure hydrogen must store the hydrogen onboard as a compressed gas in pressurized tanks. Due to the low energy density of hydrogen, it is difficult to store enough hydrogen onboard to allow vehicles to travel the same distance as gasoline-powered vehicles before refueling, typically 300-400 miles. Higher-density liquid fuels such as methanol, ethanol, natural gas, liquefied petroleum gas, and gasoline can be used for fuel, but the vehicles must have an onboard fuel processor to reform the methanol to hydrogen. This increases costs and maintenance requirements. The reformer also releases carbon dioxide (a greenhouse gas), though less than that emitted from current gasoline-powered engines.
Phosphoric Acid Fuel Cell
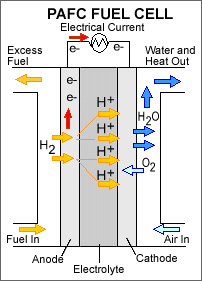 Phosphoric acid fuel cells use liquid
phosphoric acid as an electrolyte—the acid is contained in a Teflon-bonded
silicon carbide matrix—and porous carbon electrodes containing a platinum
catalyst. The chemical reactions that take place in the cell are shown in
the diagram to the right.
Phosphoric acid fuel cells use liquid
phosphoric acid as an electrolyte—the acid is contained in a Teflon-bonded
silicon carbide matrix—and porous carbon electrodes containing a platinum
catalyst. The chemical reactions that take place in the cell are shown in
the diagram to the right.
The phosphoric acid fuel cell (PAFC) is considered the "first generation" of modern fuel cells. It is one of the most mature cell types and the first to be used commercially, with over 200 units currently in use. This type of fuel cell is typically used for stationary power generation, but some PAFCs have been used to power large vehicles such as city buses.
PAFCs are more tolerant of impurities in the reformate than PEM cells, which are easily "poisoned" by carbon monoxide—carbon monoxide binds to the platinum catalyst at the anode, decreasing the fuel cell's efficiency. They are 85 percent efficient when used for the co-generation of electricity and heat, but less efficient at generating electricity alone (37 to 42 percent). This is only slightly more efficient than combustion-based power plants, which typically operate at 33 to 35 percent efficiency. PAFCs are also less powerful than other fuel cells, given the same weight and volume. As a result, these fuel cells are typically large and heavy. PAFCs are also expensive. Like PEM fuel cells, PAFCs require an expensive platinum catalyst, which raises the cost of the fuel cell. A typical phosphoric acid fuel cell costs between $4,000 and $4,500 per kilowatt to operate.
Alkaline Fuel Cell
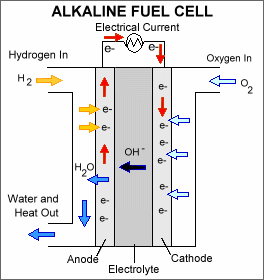 Alkaline fuel cells (AFCs) were one of
the first fuel cell technologies developed, and they were the first type
widely used in the U.S. space program to produce electrical energy and water
onboard spacecraft. These fuel cells use a solution of potassium hydroxide
in water as the electrolyte and can use a variety of non-precious metals as
a catalyst at the anode and cathode. High-temperature AFCs operate at
temperatures between 100ΊC and 250ΊC (212ΊF and 482ΊF). However, more-recent
AFC designs operate at lower temperatures of roughly 23ΊC to 70ΊC (74ΊF to
158ΊF).
Alkaline fuel cells (AFCs) were one of
the first fuel cell technologies developed, and they were the first type
widely used in the U.S. space program to produce electrical energy and water
onboard spacecraft. These fuel cells use a solution of potassium hydroxide
in water as the electrolyte and can use a variety of non-precious metals as
a catalyst at the anode and cathode. High-temperature AFCs operate at
temperatures between 100ΊC and 250ΊC (212ΊF and 482ΊF). However, more-recent
AFC designs operate at lower temperatures of roughly 23ΊC to 70ΊC (74ΊF to
158ΊF).
AFCs are high-performance fuel cells due to the rate at which chemical reactions take place in the cell. They are also very efficient, reaching efficiencies of 60 percent in space applications.
The disadvantage of this fuel cell type is that it is easily poisoned by carbon dioxide (CO2). In fact, even the small amount of CO2 in the air can affect the cell's operation, making it necessary to purify both the hydrogen and oxygen used in the cell. This purification process is costly. Susceptibility to poisoning also affects the cell's lifetime (the amount of time before it must be replaced), further adding to cost.
Cost is less of a factor for remote locations such as space or under the sea. However, to effectively compete in most mainstream commercial markets, these fuel cells will have to become more cost effective. AFC stacks have been shown to maintain sufficiently stable operation for more than 8,000 operating hours. To be economically viable in large-scale utility applications, these fuel cells need to reach operating times exceeding 40,000 hours. This is possibly the most significant obstacle in commercializing this fuel cell technology.
Molten Carbonate Fuel Cell
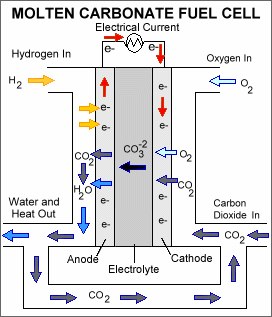 Molten carbonate fuel cells (MCFCs) are
currently being developed for natural gas and coal-based power plants for
electrical utility, industrial, and military applications. MCFCs are
high-temperature fuel cells that use an electrolyte composed of a molten
carbonate salt mixture suspended in a porous, chemically inert ceramic
lithium aluminum oxide (LiAlO2) matrix. Since they operate at extremely high
temperatures of 650ΊC (roughly 1,200ΊF) and above, non-precious metals can
be used as catalysts at the anode and cathode, reducing costs.
Molten carbonate fuel cells (MCFCs) are
currently being developed for natural gas and coal-based power plants for
electrical utility, industrial, and military applications. MCFCs are
high-temperature fuel cells that use an electrolyte composed of a molten
carbonate salt mixture suspended in a porous, chemically inert ceramic
lithium aluminum oxide (LiAlO2) matrix. Since they operate at extremely high
temperatures of 650ΊC (roughly 1,200ΊF) and above, non-precious metals can
be used as catalysts at the anode and cathode, reducing costs.
Improved efficiency is another reason MCFCs offer significant cost reductions over phosphoric acid fuel cells (PAFCs). Molten carbonate fuel cells can reach efficiencies approaching 60 percent, considerably higher than the 37-42 percent efficiencies of a phosphoric acid fuel cell plant. When the waste heat is captured and used, overall fuel efficiencies can be as high as 85 percent.
Unlike alkaline, phosphoric acid, and polymer electrolyte membrane fuel cells, MCFCs don't require an external reformer to convert more energy-dense fuels to hydrogen. Due to the high temperatures at which they operate, these fuels are converted to hydrogen within the fuel cell itself by a process called internal reforming, which also reduces cost.
Molten carbonate fuel cells are not prone to carbon monoxide or carbon dioxide "poisoning"—they can even use carbon oxides as fuel—making them more attractive for fueling with gases made from coal. Although they are more resistant to impurities than other fuel cell types, scientists are looking for ways to make MCFCs resistant enough to impurities from coal, such as sulfur and particulates.
The primary disadvantage of current MCFC technology is durability. The high temperatures at which these cells operate and the corrosive electrolyte used accelerate component breakdown and corrosion, decreasing cell life. Scientists are currently exploring corrosion-resistant materials for components as well as fuel cell designs that increase cell life without decreasing performance.
Solid Oxide Fuel Cell
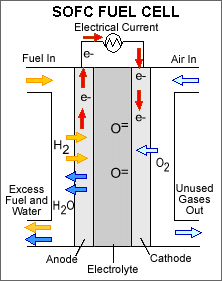 Solid oxide fuel cells (SOFCs) use a
hard, non-porous ceramic compound as the electrolyte. Since the electrolyte
is a solid, the cells do not have to be constructed in the plate-like
configuration typical of other fuel cell types. SOFCs are expected to be
around 50-60 percent efficient at converting fuel to electricity. In
applications designed to capture and utilize the system's waste heat
(co-generation), overall fuel use efficiencies could top 80-85 percent.
Solid oxide fuel cells (SOFCs) use a
hard, non-porous ceramic compound as the electrolyte. Since the electrolyte
is a solid, the cells do not have to be constructed in the plate-like
configuration typical of other fuel cell types. SOFCs are expected to be
around 50-60 percent efficient at converting fuel to electricity. In
applications designed to capture and utilize the system's waste heat
(co-generation), overall fuel use efficiencies could top 80-85 percent.
Solid oxide fuel cells operate at very high temperatures—around 1,000ΊC (1,830ΊF). High temperature operation removes the need for precious-metal catalyst, thereby reducing cost. It also allows SOFCs to reform fuels internally, which enables the use of a variety of fuels and reduces the cost associated with adding a reformer to the system.
SOFCs are also the most sulfur-resistant fuel cell type; they can tolerate several orders of magnitude more sulfur than other cell types. In addition, they are not poisoned by carbon monoxide (CO), which can even be used as fuel. This allows SOFCs to use gases made from coal.
High-temperature operation has disadvantages. It results in a slow startup and requires significant thermal shielding to retain heat and protect personnel, which may be acceptable for utility applications but not for transportation and small portable applications. The high operating temperatures also place stringent durability requirements on materials. The development of low-cost materials with high durability at cell operating temperatures is the key technical challenge facing this technology.
Scientists are currently exploring the potential for developing lower-temperature SOFCs operating at or below 800ΊC that have fewer durability problems and cost less. Lower-temperature SOFCs produce less electrical power, however, and stack materials that will function in this lower temperature range have not been identified.
Direct Methanol Fuel Cell
Most fuel cells are powered by hydrogen, which can be fed to the fuel cell system directly or can be generated within the fuel cell system by reforming hydrogen-rich fuels such as methanol, ethanol, and hydrocarbon fuels. Direct methanol fuel cells (DMFCs), however, are powered by pure methanol, which is mixed with steam and fed directly to the fuel cell anode.
Direct methanol fuel cells do not have many of the fuel storage problems typical of some fuel cells since methanol has a higher energy density than hydrogen—though less than gasoline or diesel fuel. Methanol is also easier to transport and supply to the public using our current infrastructure since it is a liquid, like gasoline.
Direct methanol fuel cell technology is relatively new compared to that of fuel cells powered by pure hydrogen, and research and development are roughly 3-4 years behind that of other fuel cell types.
Regenerative (Reversible) Fuel Cells
Regenerative fuel cells produce electricity from hydrogen and oxygen and generate heat and water as byproducts, just like other fuel cells. However, regenerative fuel cell systems can also use electricity from solar power or some other source to divide the excess water into oxygen and hydrogen fuel—this process is called "electrolysis." This is a comparatively young fuel cell technology being developed by NASA and others.
![]()
|
FC Technology |
PAFC |
PEMFC |
SOFC |
AFC |
MCFC |
|
Electrolyte |
Phosphoric acid |
Proton exchange membrane |
Solid Metal Oxide |
Potassium Hydroxide (KOH) |
Alkaline Carbonates (CO3) |
|
Operating temperature |
150-200oC |
80oC |
800-1000oC |
150-200oC |
650oC |
|
Electrical efficiency |
35-45(%) |
42-50(%) |
45-60(%) |
40(%) |
45-55(%) |
|
Power output |
200KW |
<250KW |
100kW |
300W - 5KW |
250KW - 2MW |
|
Applications |
Buses Large Consumers Remote Consumers |
Transport Small Devices Small Consumers |
Medium to large scale power generation |
Aerospace Transport |
Large Consumers |
|
Poisons |
CO(<0.5%) S(<50ppm) |
CO(50ppm per stack) |
S(<1.0ppm) |
CO,CH4, CO2,H2O,S |
S(<1.0ppm) |
|
Pros |
|
|
|
|
|
|
Cons |
|
|
|
|
|
![]()
Most fuel cells systems use pure hydrogen or
hydrogen-rich fuels, such as methanol, gasoline, diesel, or gasified coal,
to produce electricity. Both fuel types have advantages and
limitations.
Pure Hydrogen
Most fuel cell systems are fueled with pure hydrogen gas, which is stored onboard as a compressed gas. Since hydrogen gas has a low energy density, it is difficult to store enough hydrogen to generate the same amount of power as with conventional fuels such as gasoline. This is a significant problem for fuel cell vehicles, which need to have a driving range of 300-400 miles between refueling to be competitive gasoline vehicles. High-pressure tanks and other technologies are being developed to allow larger amounts of hydrogen to be stored in tanks small enough for passenger cars and trucks.
In addition to onboard storage problems, our current infrastructure for
getting liquid fuel to consumers can't be used for gaseous hydrogen. New
facilities and delivery systems must be built, which will require
significant time and resources. Costs for large-scale deployment will be
substantial.
Hydrogen-rich Fuels
Fuel cell systems can also be fueled with hydrogen-rich fuels, such as methanol, natural gas, gasoline, or gasified coal. In many fuel cell systems, these fuels are passed through onboard "reformers" that extract hydrogen from the fuel. Onboard reforming has several advantages:
- It allows the use of fuels with higher energy density than pure hydrogen gas, such as methanol, natural gas, and gasoline.
- It allows the use of conventional fuels delivered using the existing infrastructure (e.g., liquid gas pumps for vehicles and natural gas lines for stationary source).
There are also several disadvantages to reforming hydrogen-rich fuels:
- Onboard reformers add to the complexity, cost, and maintenance demands of fuel cell systems.
- If the reformer allows carbon monoxide to reach the fuel cell anode, it can gradually decrease the performance of the cell.
- Reformers produce carbon dioxide (a prominent greenhouse gas) and other air pollutants, but less than typical fossil combustion processes.
High-temperature fuel cell systems can reform fuels within the fuel cell
itself—a process called internal reforming—removing the need for onboard
reformers and their associated costs. Internal reforming, however, does emit
carbon dioxide, just like onboard reforming. In addition, impurities in the
gaseous fuel can reduce cell efficiency.
Fuel Cell Systems
References:
[1] U.S. Department of Energy: http://www.energy.gov/engine/content.do?BT_CODE=ES_HYDROGEN
[2] National Fuel Cell Research Center: http://www.nfcrc.uci.edu/fcresources/FCexplained/FC_Types.htm