|
|
|
|
|
|
|
|
|
|
The Hydrogen Storage Problem
Hydrogen is the most abundant element in the universe and has great potential as an energy source. The main drawback towards its commercialization though is focused on how it can be stored effectively, so it meets predetermined standards of safety. One gram of hydrogen occupies about 11 liters (2.9 gallons) of space at atmospheric pressure and also possesses the ability to escape easier from tanks in the atmosphere than any other known gas. All this facts denote the 'fragile' character of hydrogen and highlight the need of a dynamically designed storing infrastructure for a filling station that takes the factors of cost efficiency, safety, operational efficiency and performance under its primary considerations.
Hydrogen Storage Techniques
There is a vast range of techniques available for hydrogen storing at the current time. A few of them are already operational, while the remaining ones are in the research and development stage with the anticipation to become commercial in the next ten years. A comprehensive presentation of these techniques is performed below:
· Metal Hydride tanks
Metal Hydrides are specific combinations of metallic alloys that operate according to the sponge-absorbing-water principle. They possess the ability to absorb hydrogen and release it at a second stage, either at room temperature or through the application of heat to the tank. The amount of hydrogen absorbed varies from 1-2 % of the total weight of the tank. Some hydride tanks can store 5-7 % of their own weight but only when exposed to a lower heating temperature limit of 250 °C.
Advantages and Drawbacks:
Metal Hydrides offer the advantage of
safely delivering hydrogen at a constant pressure. Their working life is directly
proportional to the purity of the hydrogen stored. The alloy, during the absorption
process, acts as a sponge that can easily absorb impurities as well as the hydrogen
it is storing. As a result hydrogen is released from the tank eventually in
an extremely pure form but the impurities are left behind filling the spaces
in the metal that the hydrogen once occupied decreasing the tank's lifetime.
Hydrid Model BL-120
Stainless Steel Pressure Vessel |
Hydrogen Capacity: 120 std. liters |
Hydride Mass: 907 grams |
Container Mass * : 160 grams |
Total Mass: 1067 grams |
Diameter: 2.25 inches |
Height: 5 inches |
· Chemically stored hydrogen
It is a fact that hydrogen, due to its elemental character, can be found in numerous chemical compounds. Many of these compounds are utilized as a hydrogen storage method. A stable compound containing the hydrogen is created by the combination of hydrogen in a chemical reaction. A secondary reaction also is taking place aiming to release the hydrogen which is later collected and utilized by a fuel cell. The exact reaction employed varies according to the storage compound type.
Advantages and Drawbacks:
Ammonia cracking, partial oxidation and methanol cracking consist some of the techniques applied. Their advantage is focused on eliminating the need for a storage unit for the hydrogen produced because the latter is produced on demand. Although this technology looks promising it is still in the research and development stage.
Anode off-gas Heated Cracker Prototype
· Liquid Carrier Storage
The term is used to clarify the hydrogen being stored in the fossil fuels that are common today. Whenever natural gas methanol or gasoline is utilized as hydrogen's sources, the fossil fuel requires undergoing through a reforming process. The aim of the process is to remove the hydrogen from the original fossil fuel. At a second stage, excess carbon monoxide; a substance that can poison certain types of fuel cell; is removed from the reformed hydrogen. The cleaned and reformed hydrogen can now be utilized by the fuel cell.
Advantages and Drawbacks:
Reformers are currently at the secondary phase
of laboratory testing. Many companies are also operating or designing
prototypes.
The Me-100 Hydrogen Generator makes ultra-pure H2 from Methanol and Water.
· Carbon nanotubes
A designation given for microscopic tubes of carbon with dimension of two nanometers across, which are able of storing hydrogen in the microscopic pores and within the tube structures. The principle of hydrogen release when heat provided is also applicable in carbon nanotubes as it is with metal hydrides. The advantage of carbon nanotubes is focused in the quantity of hydrogen stored per % of their own weight (4.2-65).
Advantages and Drawbacks:
Carbon nanotubes clearly possess the advantage of the highest hydrogen quantity storing ability per % of their own weight. Unfortunately, they still are in the primary stages of research and development with an anticipation to become commercial in fifteen years from now.
Carbon Nanotubes types: a (5,
5) armchair nanotube (top), a (9, 0) zigzag nanotube (middle) and a (10,
5) chiral nanotube
· Glass Microspheres
Glass Microspheres are configurations of tiny glass spheres which are
used to safely store hydrogen. The process of capturing the hydrogen is
quite complex and is comprised by a number of stages. Initially, the glass
spheres are warmed resulting the permeability increase of their walls.
In the next stage, they are immersed in high pressure hydrogen gas. Finally,
the spheres are cooled, 'locking' the hydrogen inside them until a subsequent
temperature increase will permit the release of hydrogen.
Advantages and Drawbacks:
Safety, avoidance of contamination and satisfactory performance under a high pressured gas environment clarify the potential of Glass Microspheres as effective hydrogen storage media. They are still in research and development stages and the overall process is considered very expensive for commercialization now.
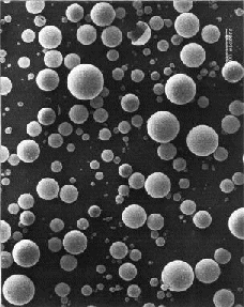
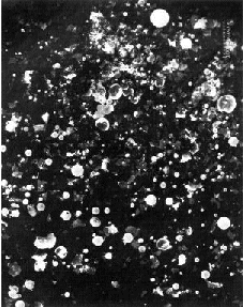
SEM Glass Microspheres Before Pressure SEM Glass Microspheres After Pressure
· Liquid Hydrogen:
Hydrogen can exist in liquid state in extremely low temperatures of 20 K (-253 °C). Liquid hydrogen requiring the additional application of compression is stored in cryogenic tanks. The cooling and compressing process consume energy resulting in a net loss of about 30 % of the energy that the liquid hydrogen is storing. The storage tanks are reinforced and insulated in order to withstand the pressure applied and to preserve temperature levels.
Advantages and Drawbacks:
Already tested technology; operating in a number of experimental hydrogen filling stations in Europe and the USA. Overall safety is maintained by preserving the Kelvin temperature and the pressure levels constant. The liquefaction gives the advantage of shrinking the hydrogen's volume 1000 times in the cryogenic storage tank. The combination though of energy needed for transforming the hydrogen to its liquid state and energy required in sustaining the pressure and the temperature in the cryogenic tank in relation to costs results a very expensive process comparative to other methods of hydrogen storing. Still, research in the field of liquid hydrogen storage is performed focusing in the development of composite tank materials and improved methods of liquefaction.
Liquid Hydrogen Filling Station in Berlin
· Compressed Hydrogen:
Hydrogen can be compressed into high-pressure tanks in a gas form. The particular process demands energy in order to achieve the compression. The space occupied by the compressed gas is large and that automatically results a lower energy density. Compression vessels should be regularly tested to ensure no leaks are taking place.
Advantages and Drawbacks:
The Compressed Hydrogen technology's main cost
is focused in the capital cost of building the pressure vessel and acquiring
the compressor. In that essence, no additional expenses are expected to
be made; rather than electricity costs to drive the compressor. Comparing
these values with the expenses required for the Liquid Hydrogen storing
technique; it can be easily justified the Compressed Hydrogen storing
technology is more cost-efficient than the Liquid Hydrogen storing technology.
Furthermore, it involves a less infrastructure and it has been operating
in a number of experimental hydrogen filling stations in Europe and the
USA.
Compressed Hydrogen Vessels