
lead to serious illness
 |
| Traffic pollution can lead to serious illness |
In recent Years there has been widespread and increasing concern with regards to reducing global emissions of greenhouse
gases, notably carbon dioxide, sulphur dioxide and nitrous oxides.
Historically, heavy industry and power stations were the principle emitters of these gases. However within the last
decade significant amounts of money have been spent by industry to reduce emissions of sulphur dioxide and nitrous oxides.
The UK Governmentís air quality statistics confirm that the major threat to clean air and urban air quality is now
coming from the road transport industry and in particular from carbon monoxide, carbon dioxide, nitrous oxides and
particulate matter.
The combustion of petrol and diesel produces many different types of local air pollutants. The use of biofuels may result in reductions in emissions of these gases. More information regarding the emisions performance of biofuels in comparison to traditional fossil fuels can be found here. There are seven main types of local air pollution. What follows is a brief description of each of these pollutants and a brief mention to acid rain.
Carbon Monoxide (Co)
Carbon monoxide is a colourless, odourless and toxic gas, which if inhaled in large quantities can interfere with the
human bodyís ability to transport oxygen to vital organs including the heart and lungs.
Carbon monoxide gas is produced by the incomplete combustion of hydrocarbon fuels (petrol and diesel) in vehicle engines.
The carbon monoxide gas is then oxidised to carbon dioxide gas, which reacts with water particles in the upper atmosphere
to produce carbonic acid (acid rain).
Nitrous Oxides (Nox)
Nitrous oxide gases can irritate the lungs, lower the human bodyís resistance to respiratory diseases and infections
including influenza (especially in young children).
Nitrous oxide gases are produced from the nitrogen in the vehicle fuel reacting with the engines combustion air (Fuel NOx).
It is also produced from the high temperature reaction at 1200C of nitrogen and oxygen present in the combustion air
(Atmospheric NOx).
Nitrous oxides react with water particles in the upper atmosphere to produce nitric acid (acid rain).
Ozone (O3)
Ozone is a naturally occurring gas, however, it is also produced from photochemical reactions between sunlight and
volatile organic compounds and nitrogen dioxide gases emitted from petrol engine vehicles.
Ozone levels are highest in the summer months due to the long hours of bright sunlight and high ambient temperatures,
which increase the rate of ozone production.
The inhalation of ozone can irritate the eyes and nose and in large doses can damage the airway lining.
Particulate Matter (Pm10)
PM10 particulate matter is defined as the percentage of particulates in the air with a size of less than 10um.
The principle source of particulate matter in UK cities is from diesel fuel vehicles.
These particles are small enough to be able to penetrate deep into the lungs and can cause irritation, worsening of
pre-existing heart and lung disease and can also cause cancer due to the carcinogenic compounds that are contained
within vehicle fuels.
Particles larger than 10um are not readily inhaled and possess lessor risks to human health.
Sulphur Dioxide (So2)
Sulphur dioxide can affect human health and in particular people suffering from asthma and chronic lung diseases.
The introduction of ultra low sulphur diesel (ulsd) has reduced the amount of sulphur dioxide emissions from vehicles.
The sulphur dioxide produced from industry and power stations reacts with water particles in the upper atmosphere to
produce sulphuric acid (acid rain).
Toxic Organic Micropollutants (Tompís)
Tompís can cause a wide range of side effects from cancer to central nervous system disorders.
Tompís include polyaromatic hydrocarbons, polychlorinated biphenols, dioxins and furans, which are produced by the
incomplete combustion of hydrocarbon fuels in vehicle engines.
Volatile Organic Compounds (Vocís)
The possible health affects of breathing VOCís are cancer, central nervous system disorders, liver and kidney damage
and birth defects.
The two main volatile organic compounds present in vehicle fuels are benzene (C6H6) and 1,3-butadiene (C4H6).
Benzene is present in exhaust gas emissions as part of the un-burnt fuel and also as a product of the decomposition
of other aromatic compounds.
The combustion of benzene in petrol engines accounts for 70% of all benzene emissions. The National Air Quality
Strategy sets a target of 16.25ug/m3 of benzene in the atmosphere.
1,3-butadiene is produced from the combustion of olefins contained within the petrol and diesel fuels and the National
Air Quality Strategy sets a target of 2.25ug/m3 of butadiene in the atmosphere.
 |
| Acid rain can seriously damage ecology |
Acid Rain
Acid rain is produced when nitrous oxide, sulphur dioxide and carbon dioxide gas in the upper atmosphere react with water
particles to produce nitric acid, sulphuric acid and carbonic acid respectively.
Unpolluted rainfall normally has an acidic pH of about 5.6, whilst acid rain has a pH of between 4.1 to 5.1.
There is considerable evidence that acid rain produced from the burning of fossil fuels in the United Kingdom has led to
pollution of rivers and lakes in Scandinavia.
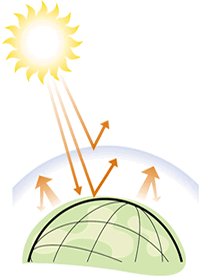 |
| Heat is trapped in the atmosphere |
Global air pollution comes in the form of green house gases. The principal greenhouse gases are chlorofluorocarbons (CFC's), carbon dioxide, methane, nitrous oxides, ozone and sulphur dioxide. These gases trap infrared radiation (heat) emitted from the earth, preventing it from escaping into space. The result of this is an increase in the atmospheric temperature. This heat trapped in the earthís atmosphere has allegedly led to the gradual melting of polar icecaps and subsequent rise in sea levels around the World as well as exaggerating the El NiŮo effect. With regard to carbon dioxide biofuels are certainly beneficial. Due to their 'carbon nutrality' these fuels produce no net output of carbon dioxide as when they grow they absorb the same amount of carbon dioxide as when they are burned. This is not entirly accurate as processes like fertilizer production and transesterification require energy and this invariably results in an output of greenhouse gases. Despite this there are still GHG savings to be made and so biofuels could help the UK meet its Kyoto Commitments.
Kyoto Protocol
The Rio de Janeiro (1991) and Kyoto (1997) Earth Summits have led to international agreements for industrialised countries
to limit and wherever possible to reduce their emissions of six principle greenhouse gases.
The six greenhouse gases are carbon dioxide, methane, nitrous oxide, hydrofluorocarbons, perfluorocarbons and sulphur
hexafluoride.
The United Kingdom has agreed to reduce these greenhouse gas emissions by 12.5% on 1990 levels between 2008 and 2012.
In addition to this agreement, the UK has also agreed to reduce emissions of carbon dioxide by 20% on 1990 levels by 2010.