Glycerine
What is Glycerine?
Glycerine is a colourless, odourless, hygroscopic, and sweet-tasting viscous
liquid. The terms glycerin, glycerine, and glycerol are often used interchangeably
in the literature, but its IUPAC official name is propane-1,2,3-triol.
The molecule can be represented as:
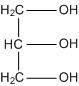
It has three hydrophilic alcoholic hydroxyl groups (OH-) that are responsible for its solubility in water.
It is hygroscopic (i.e., it absorbs water from the air); it melts at 17.8°C, boils with decomposition at 290°C, and is miscible with water and ethanol. The glycerine can be burned, but unless it's properly combusted at high temperatures it will release toxic acrolein fumes, which are mainly formed between 200 and 300 °C.
Traditional Applications
Glycerine has a wide range of applications such as an emulsifier, softening
agent, plasticizer, stabilizer and moistening agent in baked goods, ice cream,
and tobacco; in skin lotions, mouthwashes, and in innumerable pharmaceutical
and other cosmetic preparations; as a protective medium for freezing red blood
cells, sperm, corneas, and other tissues; in printing inks and in the gums
and resins in paints and coatings; in antifreeze mixtures; and as a raw material
for nitroglycerin.
Back to top
Impact of Glycerine production as biodiesel by-product
Many articles and reports address the problem of glycerine glut.
Glycerine is mainly produced as a by-product in soaps and oleochemicals production, and more recently, biodiesel. As mentioned in Biodiesel Magazine of September 2006:
“In addition, the growing oleochemical industry in Asia is producing glycerine by the barge-load. Much of it had been exported to the United States, but with rising freight costs, a majority of it is now shipped to China.
Meanwhile, the U.S. synthetic glycerine market has taken quite a beating. Synthetic glycerin is petroleum-based, where natural glycerine—such as that produced during biodiesel production—is created from fats and oils. Dow Chemical was once the nation’s only producer of synthetic glycerine. It closed its Freeport, Texas, plant in January, citing—in part—the flood of glycerine from biodiesel production. Dow Chemical still operates a glycerine plant in Germany.”
The impact of the glycerine produced as a by-product of biodiesel is not negligible.
As can be depicted from the graph below, the price of glycerine in United States plunged from 100 US$ cents/lb in 1995 to less than 40 US$ cents/lb in 2005; in Europe, the price dropped from around 1500 EUR/t to less than 500 EUR/t.
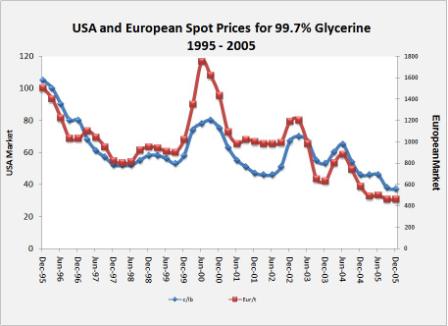 |
Source: GLYCERINE MARKET REPORT, Oleoline.com, December
2005 [19] |
Likewise, the increase in stocks of glycerine both in United States and Europe is clearly shown in the graphs below.
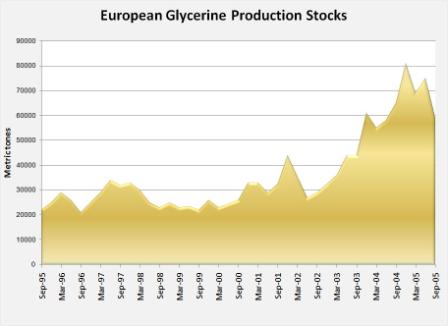 |
| Source: GLYCERINE MARKET REPORT, Oleoline.com, December 2005 [19] |
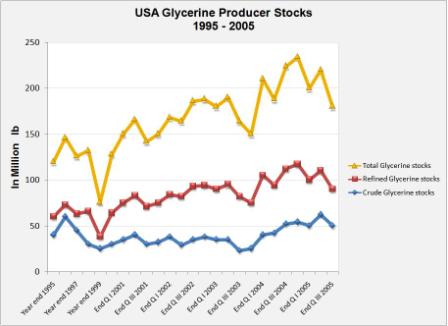 |
| Source: GLYCERINE MARKET REPORT, Oleoline.com, December 2005 [19] |
Back to top







