Hydrogen
Introduction
Many Renewable Energy Systems (RES) can produce electricity, but it is often limited by the intermittent character of solar radiation or wind speed. Hydrogen can be produced from a variety of available renewable energy resources. The potential of producing Hydrogen using these resources is analysed.
Description
Hydrogen is the worlds most abundant element and the lightest in the periodic table. Hydrogen is a high quality, low polluting fuel that can be used with high efficiency for transportation, heating, and power generation. Hydrogen is colourless, odourless and non-toxic. Moreover, when burned it does not produce carbon dioxide (CO2), carbon monoxide (CO), sulphur dioxide (SOx) or volatile organic compounds. The only by-products of hydrogen combustion are water and a very small amount of nitrogen oxide (NOx) that can be reduced to zero. Hydrogen used as main energy could offer an answer to the threat of global climate change and avoid undesirable factors associated with the use of fossil fuels.
Hydrogen is an ideal energy carrier and it can be used (in gas or liquid form) to store and transmit energy and can be used to generate electricity.
Production
Currently, most hydrogen is produced by the conversion of natural gas, using steam. This process also produces carbon dioxide, one of the main greenhouse gases, which is unwanted.
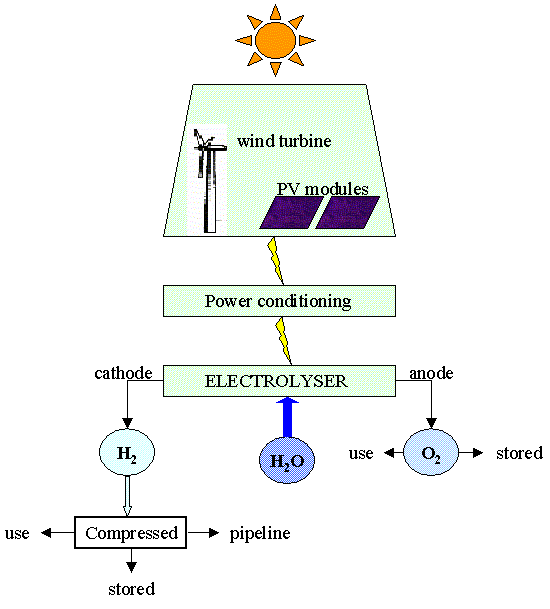
The electrolysis of water, powered by RES would produce only hydrogen and oxygen, avoiding the emission of CO2. When hydrogen is produced from the sun or others renewable energy sources it is called "solar" hydrogen. Wind, photovoltaics (PV), solar thermal and hydropower are all efficient sources of the electricity required for the electrolysis of water. The overall potential for hydrogen production from wind and solar resources is huge and we have established that a wind turbine may be a feasible source of electricity on an offshore platform, although the irregular power output from a wind turbine would have to be matched with the input requirements of the electrolyser.
Electrolysis of water
Technology for the electrolysis of water already exists and is currently on the market. It is being developed for use with intermittent power sources such as wind and solar. When DC electricity is passed between 2 electrodes (anodes and cathode) immersed in water, hydrogen collects at the negatively charged cathode and oxygen collects at the positively charged anode. The electrolysers which are commercially available are based on alkaline water electrolysis. They have a bipolar or unipolar configuration and the main chemical reactions occurring at the two electrodes are:
- Anode : 2 H2O ---> O2 + 4 H+ + 4 e-
- Cathode : 2 H+ + 2 e- ---> H2
- Global reaction : 2 H2O ---> 2 H2 + O2
Example
5 MWh of electricity would produce 1000 m3 of H2 and 500 m3 of O2.
1000 m3 of H2 will generate 1MWh of electricity and 1MWh of heat.
Sketch of the process
Electrolysis of seawater
The use of conventional electrodes, under normal conditions, for seawater electrolysis will result in the formation of chlorine in the form of sodium hypochlorite.
Chlorine is a green, highly reactive gas with a strong and irritating odour. Chlorine is used in water treatment and in the manufacture of PVC. It can be harmful and very dangerous for people and the environment and in this project we were interesting in processes which were environmentally benign.
It has been demonstrated that manganese dioxide coated electrodes are capable of producing oxygen with 99% efficiency
therefore the direct electrolysis of seawater in this way to produce hydrogen and oxygen is advantageous.
Transmission
Hydrogen is a near ideal energy transport medium. The transmission of hydrogen by pipeline is comparable to the present system of transmitting natural gas. Extensive testing indicates that natural gas pipe networks can carry a mixture of up to 20% hydrogen with no modification. The same diameter pipe can be used but at the expense of higher compressor energy (+50%). There are however problems with 'Hydrogen cracking' of the pipes, a technical problem which requires further analysis.
For long distance of transmission it was claimed that transmitting hydrogen in a pipe was cheaper than transmitting electricity in overhead cables; by around 4 times.
Storage
Many methods are available for storing hydrogen. The most common methods are:
- Hydrogen as a compressed gas: use of a pressure vessel when small amounts are stored. This technology is available but becomes expensive for large amounts.
- Hydrogen absorbing materials (metal hydrides).
- Liquid storage: well know technology (NASA). However, it is costly and requires a lot of energy to be converted in liquid.
- Underground storage: depleted oil or gas reservoirs, aquifers, salt cavities.
The choice of the storage medium for a particular application will be determined by a function of the volume to be stored and the frequency with which the store is to be (dis)charged.
Utilisation
Today, hydrogen is primarily used by industry in the manufacture of ammonia, oil refining (it is used in the process to convert crude oil into petrol) and in the synthesis of methanol. It is also used in power stations as a coolant in alternators, as well as having other uses in the chemical and pharmaceutical industry. Hydrogen is used in NASA's space program as a fuel for their space shuttles as well as in fuel cells that provide heat, electricity and drinking water for the astronauts.
Hydrogen produced by the electrolysis of water can be used either as an energy carrier (fuel or transmission) or as a storage medium. It is combusted and used as a fuel, in the similar way to petrol, in transportation: in public buses or in cars (in Germany for example). Alternatively, fuel cells could be used to power electric buses and cars (NECAR IV): a fuel cell uses reverse electrolysis where hydrogen and oxygen are used to produce electricity. It is a very efficient process (up to 70%) and the only by-products are water and heat and small amount of NOx.
Economics
Electrolysis is a well-established technology but for cost reasons is used mainly in small plant, however the hydrogen produced by electrolysis is attractive because it is very pure. The cost of hydrogen depends on the cost of the renewable energy source and the cost of transportation (in pipelines or ships). The hydrogen produced by electricity from renewable energy is very expensive at the moment. Hydrogen from electrolysis is ten times as expensive as natural gas and three times more expensive than gasoline.The most cost-effective way to produce hydrogen today is by using methane with a process called "steam reforming".
Environmental aspects
Smog, global warming, greenhouse gases, ozone depletion and acid rain are effects that have been attributed to an increase in carbon dioxide ad pollutants emission in the atmosphere essentially caused by the combustion of fossil fuels. Unlike fossil fuels, hydrogen when combusted with air does not produce pollutants or carbon dioxide. Although it is estimated that hydrogen is more expensive than fossil fuels it avoids the cost with environment and health problems associated with fossil fuels. Hydrogen made from renewable energy resources is a virtually inexhaustible, environmentally benign energy source that could meet most of our future energy needs. It's more versatile and has more uses than electricity.
Other facts about hydrogen
- Safety: research and demonstrations have shown that under everyday conditions, hydrogen is safer than all of the other fuels that are available, liquid or gas, whether used in transportation or stationary uses.
- Hydrogen has a considerable potential to overcome the limitation of intermittent renewable energy sources (solar, wind) and can therefore benefit the development of them.
- At present the use of hydrogen as storage medium for solar power utilisation is at the research and development stage.
- Hydrogen is one of the most promising energy carriers for the future.
- Technological improvements in renewable electricity could make the production of hydrogen by electrolysis very attractive for the future.
- Hydrogen is a long term option: production, storage and distribution facilities must improve and be developed.
- Many countries such as USA, Canada, Germany, are investing money in the development of hydrogen.
Conclusion
In this review we have established that there are many positive aspects of Hydrogen as a fuel and as an energy carrier. Ongoing development is increasing its market potential by reducing cost and dealing with technical problems. A Hydrogen economy will emerge in the medium term to long term. In the near future though, the demand for Hydrogen will increase due to the new EEC clean fuel regulations being introduced in the year 2000. These will require refineries to include more Hydrogen in fuel in order to decrease CO2 emissions. Oil refiners have been advised to consider investing in their own hydrogen manufacturing plants and we believe that the potential of producing hydrogen on offshore installatons using Renewable Energy Resources should be investigated further. This could give offshore operators valuable experience in Hydrogen Research & Development which could give them commercial advantage in this emerging economy. As there are no CO2 emissions from this process, this could also contibute to the reduction of emissions by offshore operators whcih is covered by the Kyoto Agreement.
