|
|
|
|
|
|
Fuel cells concerned the scientists for more than 165 years. It was the year 1839 when the Welsh justice and physician Sir William Robert Grove (1811-1896) won renown for his development of an improved wet-cell battery in 1838. The "Grove cell," as it came to be called, used a platinum electrode immersed in nitric acid and a zinc electrode in zinc sulfate to generate about 12 amps of current at about 1.8 volts [1]. This prototype consisted of two platinum electrodes which were separately surrounded by a glass cylinder. One of the cylinders was filled with hydrogen the other with oxygen. Both electrodes were immersed in diluted sulphuric acid -which was the electrolyte- and created the electric connection. At the electrodes voltage was produced. This voltage was very low and therefore Grove linked several of these fuel cells to get a higher voltage [2]. The sealed containers held water as well as the gases, and he noted that the water level rose in both tubes as the current flowed [1].

Sir William Robert Grove

Grove's Experiement
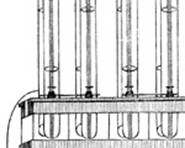
Grove's drawing of one of his experimental "gas batteries" from an 1843 letter
In 1800, British scientists William Nicholson and Anthony Carlisle had described the process of using electricity to decompose water into hydrogen and oxygen. But combining the gases to produce electricity and water was, according to Grove, "a step further that any hitherto recorded." Grove realized that by combining several sets of these electrodes in a series circuit he might "effect the decomposition of water by means of its composition." He soon accomplished this feat with the device he named a "gas battery"– the first fuel cell.
Chemist Ludwig Mond (1839 -1909) spent most of his career developing industrial chemical technology such as soda manufacturing and nickel refining. In 1889, Mond and assistant Carl Langer (d. 1935) described their experiments with a hydrogen-oxygen fuel cell that attained 6 amps per square foot (measuring the surface area of the electrode) at 0.73 volts. Mond and Langer's cell used electrodes of thin, perforated platinum. They noted difficulties in using liquid electrolytes, saying "we have only succeeded by using an electrolyte in a quasi-solid form, viz., soaked up by a porous non-conducting material, in a similar way as has been done in the so-called dry piles and batteries." An example given is an earthenware plate "impregnated by dilute sulfuric acid."
Friedrich Wilhelm Ostwald (1853 -1932), a founder of the field of physical chemistry, provided much of the theoretical understanding of how fuel cells operate. In 1893, he experimentally determined the interconnected roles of the various components of the fuel cell: electrodes, electrolyte, oxidizing and reducing agents, anions, and cations.
Grove had speculated that the action in his gas battery occurred at the point of contact between electrode, gas, and electrolyte, but was at a loss to explain further. Ostwald, drawing on his pioneering work in relating physical properties and chemical reactions, solved the puzzle of Grove's gas battery. His exploration of the underlying chemistry of fuel cells laid the groundwork for later fuel cell researchers.
William W. Jacques (1855 -1932) was an electrical engineer and chemist. In 1896, he "startled the scientific world and general public," according to one scientist of the day, "by his broad assertion that he had invented a process of making electricity directly from coal." Jacques constructed a "carbon battery" in which air was injected into an alkali electrolyte to react (he thought) with a carbon electrode (see image at right). It turned out, however, that instead of electrochemical action with an efficiency of 82 percent, he was obtaining thermoelectric action with an efficiency of about 8 percent.
Emil Baur (1873 -1944) of Switzerland (along with several students at Braunschweig and Zurich) conducted wide-ranging research into different types of fuel cells during the first half of the twentieth century. Baur's work included high temperature devices (using molten silver as an electrolyte) and a unit that used a solid electrolyte of clay and metal oxides.
Francis Thomas Bacon (1904 -1992) began researching alkali electrolyte fuel cells in the late 1930s. In 1939, he built a cell that used nickel gauze electrodes and operated under pressure as high as 3000 psi. During World War II, Bacon worked on developing a fuel cell that could be used in Royal Navy submarines, and in 1958 demonstrated an alkali cell using a stack of 10-inch diameter electrodes for Britain's National Research Development Corporation. Though expensive, Bacon's fuel cells proved reliable enough to attract the attention of Pratt & Whitney. The company licensed Bacon's work for the Apollo spacecraft fuel cells [1].
Except from these few scientists, the importance of Groves`s discovery was underestimated by contemporaries and the fuel cell was forgotten. Only in the 1950`s, against the background of the Cold War, his idea was taken up again. Space travel and military technology required compact and powerful energy sources. Spacecraft and submarines require electric power and it is not possible to work with internal combustion engines [2].
In 1960's, a new government agency was about to undertake the first step in maturing fuel cell technology. The National Aeronautics and Space Administration (NASA) was developing the mission critical systems for the first prolonged manned flight into space. Once in space, the orbiter needed a source of electricity. Batteries were ruled out due to the size, weight and toxicity necessary to support a mission of eight days in space. Photovoltaics were not practical, at the time, due to the size and weight of the solar panels necessary. The once obscure fuel cell became the technological solution to NASA's dilemma of how to provide power for extended missions to space. The earlier problems of cost and fuel supplies that plagued fuel cells became irrelevant as the spacecraft was already carrying liquid hydrogen and oxygen. An additional benefit of fuel cells over other technology was that the astronauts could consume the fuel cell's water by-product. On the early missions powered by fuel cells, there were problems with the systems that required attention. On each subsequent mission the fuel cells became increasingly reliable and today NASA's space shuttle relies on fuel cells for electricity and drinking water once in orbit.
NASA and the space program provided fuel cells with the initial research and development the technology required. Since their adoption by the space program, fuel cell technology has achieved widespread recognition by industry and government as a clean energy source for the future. With this in mind, the amount of interest in fuel cells has expanded exponentially to where 8 of the 10 largest companies in the world are involved with fuel cells in some respect. Today, billions of dollars have been spent on research and the commercialization of fuel cell products. Over the next couple of years, the products that have been in the commercialization process will begin to be available to consumers [3].
The civil use of fuel cells became interesting only during the last years. At the beginning of the 90`s scientists and engineers developed different new concepts and technologies which made it possible to increase efficiency continually and to decrease costs at the same time. Today fuel cells can be used for a lot of different applications: for vehicle engines, for residential heating systems and also for big power stations with a power rating of several megawatts as well as for smallest applications like in mobile phones or mobile computers[2].
References:
[2] http://www.hynet.info/hydrogen_e/fuelcells/main_01.html
[3] http://www.fuelcellstore.com/information/fuel_cell_history.html

